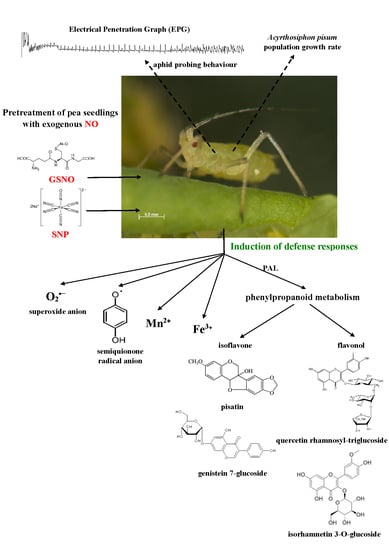
The Dynamics of the Defense Strategy of Pea Induced by Exogenous Nitric Oxide in Response to Aphid Infestation
Abstract
:1. Introduction
2. Results
2.1. The Effect of Nitric Oxide Donors on Demographic Parameters of Acyrthosiphon pisum Population
2.2. Effect of Exogenous Nitric Oxide on Behavioral Responses of A. pisum during Probing in Plant Tissues of Pea
2.3. The Effect of Nitric Oxide Donors and A. pisum on Semiquinone Radical Generation
2.4. The Effect of Nitric Oxide Donors and A. pisum on the Concentration of Manganese (Mn2+) and Iron (Fe3+) Ions
2.5. The Effect of Nitric Oxide Donors and A. pisum on Superoxide Anion Generation
2.6. The Effect of Nitric Oxide Donors and A. pisum on Expression Levels of Phenylalanine Ammonialyase Genes
2.7. The Effect of Nitric Oxide Donors and A. pisum on Accumulation of Pisatin
2.8. The Effect of Nitric Oxide Donors and A. pisum on the Level of Isoflavonoid and Flavonoid Glycosides
2.9. The Effect of Nitric Oxide Donors and A. pisum on β-Glucosidase Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Aphids and Experiment on Infestation
4.3. Determination of the Effect of Nitric Oxide Donors on the Demographic Parameters of A. pisum Population
- (a)
- intrinsic rate of increase, rm;
- (b)
- net reproductive value, Ro;
- (c)
- finite rate of increase, λ; and
- (d)
- mean generation time, T.
4.4. The Effect of Nitric Oxide Donors on Behavioral Responses of A. pisum during Probing and Feeding
4.5. Determination of Semiquinone Radicals and Manganese and Iron Ions
4.6. Determination of Superoxide Anion Content
4.7. Analysis of Pisatin and Flavonols
4.7.1. Isolation of Phenolic Compounds
4.7.2. Liquid Chromatography–Mass Spectrometry (LC/UV/ESI/MS/MS)
4.8. Total RNA Extraction and Semiquantitative RT-PCR Analysis
4.9. Extraction and Assay of β 1,3-Glucosidase Activity
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SNP | Sodium nitroprusside |
| GSNO | S-nitrosoglutathione |
| SNAP | S-nitroso-N-acetylpenicillamine |
| SIN-1 | 3-Morpholinosydnomine |
| +GSNO | Pretreated with GSNO and not infested by aphids |
| +SNP | Pretreated with SNP and not infested by aphids |
| −GSNO | Not pretreated with GSNO and not infested by aphids |
| −SNP | Not pretreated with SNP and not infested by aphids |
| +GSNO + aphid | Pretreated with GSNO and infested by aphids |
| +SNP + aphid | Pretreated with SNP and infested by aphids |
| −GSNO + aphid | Not pretreated with GSNO and infested by aphids |
| −SNP + aphid | Not pretreated with SNP and infested by aphids |
| NO | Nitric oxide |
| EPR | Electron paramagnetic resonance |
| fr. wt. | Fresh weight |
| dry wt. | Dry weight |
| g | Gram |
| g | The factor describing energy levels of the unpaired electron for the paramagnetic centre in an external magnetic field |
| h | Hour |
| hpi | Hours post infestation |
| ROS | Reactive oxygen species |
| O2•− | Superoxide anion |
| PAL | Phenylalanine ammonia-lyase |
| RNS | Reactive nitrogen species |
| ONOO− | Peroxynitrite |
| cADPR | Cyclic ADP ribose |
| Mn2+ | Manganese ions |
| Fe3+ | Iron ions |
| EPG | Electrical Penetration Graph |
| LC/UV/ESI/MS/MS | Liquid chromatography/ultraviolet detection/electrospray–mass spectrometry (tandem mass spectrometry) |
| 24 h +GSNO | Seedlings offered to aphids 24 h after treatment with GSNO in EPG experiment |
| 48 h +GSNO | Seedlings offered to aphids 48 h after treatment with GSNO in EPG experiment |
| 24 h +SNP | Seedlings offered to aphids 24 h after treatment with SNP in EPG experiment |
| 48 h +SNP | Seedlings offered to aphids 48 h after treatment with SNP in EPG experiment, PAMPs, Pathogen-associated molecular patterns, PTI, PAMP-triggered immunity; MAPK, Mitogen-activated protein kinase |
References
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Thalineau, E.; Truong, H.N.; Berger, A.; Fournier, C.; Boscari, A.; Wendehenne, D.; Jeandroz, S. Cross-regulation between N metabolism and nitric oxide (NO) signaling during plant immunity. Front. Plant Sci. 2016, 7, 472. [Google Scholar] [CrossRef] [PubMed]
- Kuśnierczyk, A.; Winge, P.E.R.; Jørstad, T.S.; Troczyńska, J.; Rossiter, J.T.; Bones, A.M. Towards global understanding of plant defence against aphids–timing and dynamics of early Arabidopsis defence responses to cabbage aphid (Brevicoryne brassicae) attack. Plant Cell Environ. 2008, 31, 1097–1115. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Z.; Visser, R.G.F.; Broekgaarden, C.; Vosman, B. Overexpression of IRM1 enhances resistance to aphids in Arabidopsis thaliana. PLoS ONE 2013, 8, e70914. [Google Scholar] [CrossRef]
- Thompson, G.A.; Goggin, F.L. Transcriptomics and functional genomics of plant defence induction by phloem-feeding insects. J. Exp. Bot. 2006, 57, 755–766. [Google Scholar] [CrossRef] [PubMed]
- Divol, F.; Vilaine, F.; Thibivilliers, S.; Kusiak, C.; Sauge, M.H.; Dinant, S. Involvement of the xyloglucan endotransglycosylase/hydrolases encoded by celery XTH1 and Arabidopsis XTH33 in the phloem response to aphids. Plant Cell Environ. 2007, 30, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Douillet-Breuil, A.C.; Bessis, R.; Debord, S.; Sbaghi, M.; Adrian, M. Phytoalexins from the Vitaceae: Biosynthesis, phytoalexin gene expression in transgenic plants, antifungal activity, and metabolism. J. Agric. Food Chem. 2002, 50, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Clément, C.; Courot, E.; Cordelier, S. Modulation of phytoalexin biosynthesis in engineered plants for disease resistance. Int. J. Mol. Sci. 2013, 14, 14136–14170. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P. Phytoalexins: Current progress and future prospects. Molecules 2015, 20, 2770–2774. [Google Scholar] [CrossRef]
- Morkunas, I.; Woźniak, A.; Formela, M.; Marczak, Ł.; Narożna, D.; Borowiak-Sobkowiak, B.; Kühn, C.; Grimm, B. Pea aphid infestation induces changes in flavonoids, antioxidative defence, soluble sugars and sugar transporter expression in leaves of pea seedlings. Protoplasma 2016, 253, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Inbar, M.O.; Mayer, R.T.; Doostdar, H.A. Induced activity of pathogenesis related (PR) proteins in aphid galls. Symbiosis 2003, 34, 293–300. [Google Scholar]
- De Ilarduya, O.M.; Xie, Q.; Kaloshian, I. Aphid-induced defense responses in Mi-1-mediated compatible and incompatible tomato interactions. Mol. Plant Microbe Interact. 2003, 16, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Narożna, D.; Nowak, W.; Samardakiewicz, S.; Remlein-Starosta, D. Cross-talk interactions of sucrose and Fusarium oxysporum in the phenylpropanoid pathway and the accumulation and localization of flavonoids in embryo axes of yellow lupine. J. Plant Physiol. 2011, 168, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.C.; Drzewiecka, K.; Jeleń, H.; Narożna, D.; Rucińska-Sobkowiak, R.; Kęsy, J.; Floryszak-Wieczorek, J.; Gabryś, B.; Morkunas, I. Differential induction of Pisum sativum defense signaling molecules in response to pea aphid infestation. Plant Sci. 2014, 221, 1–12. [Google Scholar] [CrossRef]
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant–pathogen interactions. Nat. Rev. Genet. 2010, 11, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Blackman, R.L.; Eastop, V. Taxonomic issues. In Aphids as Crop Pests; van Emden, H., Harrington, R., Eds.; CAB International: Wallingford, UK, 2007; pp. 1–30. [Google Scholar]
- Sanz, L.; Albertos, P.; Mateos, I.; Sánchez-Vicente, I.; Lechón, T.; Fernández-Marcos, M.; Lorenzo, O. Nitric oxide (NO) and phytohormones crosstalk during early plant development. J. Exp. Bot. 2015, 66, 2857–2868. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, M.C.; Sell, S.; Huang, X.; Scherf, M.; Werner, T.; Durner, J.; Lindermayr, C. Nitric oxide-responsive genes and promoters in Arabidopsis thaliana: A bioinformatics approach. J. Exp. Bot. 2008, 59, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Barroso, J.B. Functional implications of peroxisomal nitric oxide (NO) in plants. Front. Plant Sci. 2014, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Lamattina, L.; García-Mata, C.; Graziano, M.; Pagnussat, G. Nitric oxide: The versatility of an extensive signal molecule. Annu. Rev. Plant Biol. 2003, 54, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Besson-Bard, A.; Pugin, A.; Wendehenne, D. New insights into nitric oxide signaling in plants. Annu. Rev. Plant Biol. 2008, 59, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Courtois, C.; Besson, A.; Dahan, J.; Bourque, S.; Dobrowolska, G.; Pugin, A.; Wendehenne, D. Nitric oxide signaling in plants: Interplays with Ca2+ and protein kinases. J. Exp. Bot. 2008, 59, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, E. The language of nitric oxide signaling. Plant Biol. 2011, 13, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Lindermayr, C.; Saalbach, G.; Durner, J. Proteomic identification of S-nitrosylated proteins in Arabidopsis. Plant Physiol. 2005, 137, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Lindermayr, C.; Saalbach, G.; Bahnweg, G.; Durner, J. Differential inhibition of Arabidopsis methionine adenosyltransferases by protein S-nitrosylation. J. Biol. Chem. 2006, 281, 4285–4291. [Google Scholar] [CrossRef] [PubMed]
- Grün, S.; Lindermayr, C.; Sell, S.; Durner, J. Nitric oxide and gene regulation in plants. J. Exp. Bot. 2006, 57, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Belenghi, B.; Romero-Puertas, M.C.; Vercammen, D.; Brackenier, A.; Inzé, D.; Delledonne, M.; van Breusegem, F. Metacaspase activity of Arabidopsis thaliana is regulated by S-nitrosylation of a critical cysteine residue. J. Biol. Chem. 2007, 282, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Klessig, D.F.; Durner, J.; Noad, R.; Navarre, D.A.; Wendehenne, D.; Kumar, D.; Zhou, J.M.; Shah, J.; Zhang, S.; Kachroo, P.; et al. Nitric oxide and salicylic acid signaling in plant defense. Proc. Natl. Acad. Sci. USA 2000, 97, 8849–8855. [Google Scholar] [CrossRef] [PubMed]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J.; Kosmala, A. Are nitric oxide donors a valuable tool to study the functional role of nitric oxide in plant metabolism? Plant Biol. 2011, 13, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Weller, R. Nitric oxide: A key mediator in cutaneous physiology. Clin. Exp. Dermatol. 2003, 28, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Rőszer, T. Biosynthesis of nitric oxide in plants. In Nitric Oxide in Plants: Metabolism and Role in Stress Physiology; Springer International Publishing: New York, NY, USA, 2014; pp. 17–32. [Google Scholar]
- Floryszak-Wieczorek, J.; Milczarek, G.; Arasimowicz, M.; Ciszewski, A. Do nitric oxide donors mimic endogenous NO-related response in plants? Planta 2006, 224, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Simaei, M.; Khavari-Nejad, R.A.; Bernard, F. Exogenous application of salicylic acid and nitric oxide on the ionic contents and enzymatic activities in NaCl-stressed soybean plants. Am. J. Plant Sci. 2012, 3, 1495–1503. [Google Scholar] [CrossRef]
- Morkunas, I.; Formela, M.; Floryszak-Wieczorek, J.; Marczak, Ł.; Narożna, D.; Nowak, W.; Bednarski, W. Cross-talk interactions of exogenous nitric oxide and sucrose modulates phenylpropanoid metabolism in yellow lupine embryo axes infected with Fusarium oxysporum. Plant Sci. 2013, 211, 102–121. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.A.; Prats, E.; Pierre, S.; Hall, M.A.; Hebelstrup, K.H. Integrating nitric oxide into salicylic acid and jasmonic acid/ethylene plant defense pathways. Front. Plant Sci. 2013, 4, 215. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Carrion, A.I.; Castellano, R.; Rosales, M.A.; Ruiz, J.M.; Romero, L. Role of nitric oxide under saline stress: Implications on proline metabolism. Biol. Plant. 2008, 52, 587–591. [Google Scholar] [CrossRef]
- Arasimowicz, M.; Floryszak-Wieczorek, J. Nitric oxide as a bioactive signaling molecule in plant stress responses. Plant Sci. 2007, 172, 876–887. [Google Scholar] [CrossRef]
- Davies, S. Nitric oxide signaling in insects. Insect Biochem. Mol. Biol. 2000, 30, 1123–1138. [Google Scholar] [CrossRef]
- Müller, U. The nitric oxide system in insects. Prog. Neurobiol. 1997, 51, 363–381. [Google Scholar] [CrossRef]
- Liu, Y.B. Nitric oxide as a potent fumigant for postharvest pest control. J. Econ. Entomol. 2013, 106, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Ascenzi, P.; Gradoni, L. Nitric Oxide Limits Parasite Development in Vectors and in Invertebrate Intermediate Hosts. IUBMB Life 2002, 53, 121–123. [Google Scholar] [PubMed]
- Colasanti, M.; Venturini, G. Nitric oxide in invertebrates. Mol. Neurobiol. 1998, 17, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.M.; Nussenzveig, R.H. Nitric oxide synthase activity from a hematophagous insect salivary gland. FEBS Lett. 1993, 330, 165–168. [Google Scholar] [CrossRef]
- Paveto, C.; Pereira, C.; Espinosa, J.; Montagna, A.E.; Farber, M.; Esteva, M.; Flavia, M.M.; Torres, H.N. The nitric oxide transduction pathway in Trypanosoma cruzi. J. Biol. Chem. 1995, 270, 16576–16579. [Google Scholar] [CrossRef] [PubMed]
- Colasanti, M.; Venturini, G.; Merante, A.; Musci, G.; Lauro, G.M. Nitric oxide involvement in Hydra vulgaris very primitive olfactory-like system. J. Neurosci. 1997, 17, 493–499. [Google Scholar] [PubMed]
- Palumbo, A.; Di Cosmo, A.; Gesualdo, I. A Calcium-Dependent Nitric Oxide Synthase and NMDA R1 Glutamate Receptor in the Ink Gland of Sepia officinalis: A Hint to a Regulatory Role of Nitric Oxide in Melanogenesis? Biochem. Biophys. Res. Commun. 1997, 235, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Scholz, N.L.; Chang, E.S.; Graubard, K.; Truman, J.W. The NO/cGMP pathway and the development of neural networks in postembryonic lobsters. J. Neurobiol. 1998, 34, 208–226. [Google Scholar] [CrossRef]
- Mart, B.; Collar, J.L.; Tjallingii, W.F.; Fereres, A. Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J. Gen. Virol. 1997, 78, 2701–2705. [Google Scholar]
- Gabryś, B.; Pawluk, M. Acceptability of different species of Brassicaceae as hosts for the cabbage aphid. In Proceedings of the 10th International Symposium on Insect-Plant Relationships, Oxford, England, UK, 4–10 July 1998; Simpson, S.J., Mordue, A.J., Hardie, J., Eds.; Springer: Dordrecht, The Netherlands, 1999; pp. 105–109. [Google Scholar]
- Gabryś, B.; Tjallingii, W.F. The role of sinigrin in host plant recognition by aphids during initial plant penetration. Entomol. Exp. Appl. 2002, 104, 89–93. [Google Scholar] [CrossRef]
- Dancewicz, K.; Gabryś, B.; Dams, I.; Wawrzeńczyk, C. Enantiospecific effect of pulegone and pulegone-derived lactones on Myzus persicae (Sulz.) settling and feeding. J. Chem. Ecol. 2008, 34, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Gabryś, B.; Dancewicz, K.; Gliszczyńska, A.; Kordan, B.; Wawrzeńczyk, C. Systemic deterrence of aphid probing and feeding by novel β-damascone analogues. J. Pest Sci. 2015, 88, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, A.M.; Tjallingii, W.F.; Castanera, P. Probing behavior of Diuraphis noxia on five cereal species with different hydroxamic acid levels. Entomol. Exp. Appl. 1996, 78, 341–348. [Google Scholar] [CrossRef]
- Kordan, B.; Dancewicz, K.; Wróblewska, A.; Gabryś, B. Intraspecific variation in alkaloid profile of four lupine species with implications for the pea aphid probing behavior. Phytochem. Lett. 2012, 5, 71–77. [Google Scholar] [CrossRef]
- Dancewicz, K.; Sznajder, K.; Załuski, D.; Kordan, B.; Gabryś, B. Behavioral sensitivity of Myzus persicae to volatile isoprenoids in plant tissues. Entomol. Exp. Appl. 2016, 160, 229–240. [Google Scholar] [CrossRef]
- Alvarez, A.E.; Tjallingii, W.F.; Garzo, E.; Vleeshouwers, V.; Dicke, M.; Vosman, B. Location of resistance factors in the leaves of potato and wild tuber-bearing Solanum species to the aphid Myzus persicae. Entomol. Exp. Appl. 2006, 121, 145–157. [Google Scholar] [CrossRef]
- Marchetti, E.; Civolani, S.; Leis, M.; Chicca, M.; Tjallingii, W.F.; Pasqualini, E.; Baronio, P. Tissue location of resistance in apple to the rosy apple aphid established by electrical penetration graphs. Bull. Insectol. 2009, 62, 203–208. [Google Scholar]
- Bellin, D.; Asai, S.; Delledonne, M.; Yoshioka, H. Nitric oxide as a mediator for defense responses. Mol. Plant Microbe Interact. 2013, 26, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Pearce, R.B.; Edwards, P.P.; Green, T.L.; Anderson, P.A.; Fisher, B.J.; Carpenter, T.A.; Hall, L.D. Immobilized long-lived free radicals at the host–pathogen interface in sycamore (Acer pseudoplatanus L.). Phys. Mol. Plant Pathol. 1997, 50, 371–390. [Google Scholar] [CrossRef]
- Simontacchi, M.; Galatro, A.; Ramos-Artuso, F.; Santa-María, G.E. Plant Survival in a Changing Environment: The Role of Nitric Oxide in Plant Responses to Abiotic Stress. Front. Plant Sci. 2015, 6, 977. [Google Scholar] [CrossRef] [PubMed]
- Mai, V.C.; Bednarski, W.; Borowiak-Sobkowiak, B.; Wilkaniec, B.; Samardakiewicz, S.; Morkunas, I. Oxidative stress in pea seedling leaves in response to Acyrthosiphon pisum infestation. Phytochemistry 2013, 93, 49–62. [Google Scholar] [CrossRef]
- Zebelo, S.A.; Maffei, M.E. Role of early signaling events in plant-insect interactions. J. Exp. Bot. 2015, 66, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Kiefer, E.; von Rad, U.; Ernst, D.; Foissner, I.; Durner, J. Nitric oxide burst and nitric oxide-dependent gene induction in plants. Plant Physiol. Biochem. 2002, 40, 625–631. [Google Scholar] [CrossRef]
- Bolwell, G.P.; Wojtaszek, P. Mechanisms for the generation of reactive oxygen species in plant defence—A broad perspective. Physiol. Mol. Plant Pathol. 1997, 51, 347–366. [Google Scholar] [CrossRef]
- Moloi, M.J.; Westhuizen, A.J. Involvement of nitric oxide in the Russian wheat aphid resistance response of wheat. Cereal Res. Commun. 2013, 42, 119–125. [Google Scholar] [CrossRef]
- Foissner, I.; Wendehenne, D.; Langebartels, C.; Durner, J. In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J. 2000, 23, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Hébrard, C.; Deville, M.A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the role of phytoalexins in plant-microorganism interactions and human health. Molecules 2014, 19, 18033–18056. [Google Scholar] [CrossRef] [PubMed]
- Wendehenne, D.; Pugin, A.; Klessig, D.F.; Durner, J. Nitric oxide: Comparative synthesis and signaling in animal and plant cells. Trends Plant Sci. 2001, 6, 177–183. [Google Scholar] [CrossRef]
- Murgia, I.; de Pinto, M.C.; Delledonne, M.; Soave, C.; de Gara, L. Comparative effects of various nitric oxide donors on ferritin regulation, programmed cell death, and cell redox state in plant cells. J. Plant Physiol. 2004, 161, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.; Du, X.; Zhao, F.; Shi, R.; Wang, J. Role of nitric oxide in UV-B-induced activation of PAL and stimulation of flavonoid biosynthesis in Ginkgo biloba callus. Plant Cell Tissue Organ Cult. 2009, 97, 175–185. [Google Scholar] [CrossRef]
- Wang, J.W.; Wu, J.Y. Nitric oxide is involved in methyl jasmonate-induced defense responses and secondary metabolism activities of Taxus cells. Plant Cell Physiol. 2005, 46, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Chen, H.Y.; Zhang, H.P.; Zhu, N.; Dong, J.E. Nitric oxide triggered by salicylic acid mediates the biosynthesis of salvianolic acid B in Salvia miltiorhizza suspension cell culture. Plant Sci. J. 2015, 31, 81–89. [Google Scholar]
- Sharma, S.; Sharma, R.R. Nitric oxide inhibits activities of PAL and PME enzymes and reduces chilling injury in “Santa Rosa” Japanese plum (Prunus salicina Lindell). J. Plant Biochem. Biotechnol. 2015, 24, 292–297. [Google Scholar] [CrossRef]
- Delledonne, M.; Xia, Y.; Dixon, R.A.; Lamb, C. Nitric oxide functions as a signal in plant disease resistance. Nature 1998, 394, 585–588. [Google Scholar] [PubMed]
- Durner, J.; Wendehenne, D.; Klessig, D.F. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 1998, 95, 10328–10333. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.P. Glycosides. In Phytochemistry: The Process and Products of Photosynthesis; Miller, L.P., Ed.; Van Nostrand Reinhold: New York, NY, USA, 1973; pp. 297–375. [Google Scholar]
- Pentzold, S.; Zagrobelny, M.; Rook, F.; Bak, S. How insects overcome two-component plant chemical defence: Plant β-glucosidases as the main target for herbivore adaptation. Biol. Rev. 2014, 89, 531–551. [Google Scholar] [CrossRef] [PubMed]
- Birch, L.C. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 1948, 17, 15–26. [Google Scholar] [CrossRef]
- Wyatt, I.J.; White, P.F. Simple estimation of intrinsic rates for aphids and tetranychid mites. J. Appl. Ecol. 1977, 14, 757–766. [Google Scholar] [CrossRef]
- Kozłowski, R.; Niedbała, G.; Durak, R.; Borowiak-Sobkowiak, B. System komputerowy wspomagający wyznaczanie parametrów demograficznych i analizę populacji owadów. Tech. Rol. Ogrod. Leśna 2010, 4, 16–18. (In Polish) [Google Scholar]
- Will, T.; Tjallingii, W.F.; Thönnessen, A.; van Bel, A.J. Molecular sabotage of plant defense by aphid saliva. Proc. Natl. Acad. Sci. USA 2007, 104, 10536–10541. [Google Scholar] [CrossRef] [PubMed]
- Philippi, J.; Schliephake, E.; Jürgens, H.U.; Jansen, G.; Ordon, F. Feeding behavior of aphids on narrow-leafed lupin (Lupinus angustifolius) genotypes varying in the content of quinolizidine alkaloids. Entomol. Exp. Appl. 2015, 156, 37–51. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Sieve element acceptance by aphids. Eur. J. Entomol. 1994, 91, 47–52. [Google Scholar]
- Helden, M.; Tjallingii, W.F. Tissue localisation of lettuce resistance to the aphid Nasonovia ribisnigri using electrical penetration graphs. Entomol. Exp. Appl. 1993, 68, 269–278. [Google Scholar] [CrossRef]
- Morkunas, I.; Bednarski, W. Fusarium oxysporum-induced oxidative stress and antioxidative defenses of yellow lupine embryo axes with different sugar levels. J. Plant Physiol. 2008, 165, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Bednarski, W.; Ostrowski, A.; Waplak, S. Low temperature short-range ordering caused by Mn2+ doping of Rb3H(SO4)2. J. Phys. Condens. Matter 2010, 22, 225901. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Garnczarska, M.; Bednarski, W.; Ratajczak, W.; Waplak, S. Metabolic and ultrastructural responses of lupine embryo axes to sugar starvation. J. Plant Physiol. 2003, 160, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Bednarski, W.; Kozłowska, M. Response of embryo axes of germinating seeds of yellow lupine to Fusarium oxysporum. Plant Physiol. Biochem. 2004, 42, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Bednarski, W.; Kopyra, M. Defense strategies of pea embryo axes with different levels of sucrose to Fusarium oxysporum and Ascochyta pisi. Physiol. Mol. Plant Pathol. 2008, 72, 167–178. [Google Scholar] [CrossRef]
- Formela, M.; Samardakiewicz, S.; Marczak, Ł.; Nowak, W.; Narożna, D.; Bednarski, W.; Kasprowicz-Maluśki, A.; Morkunas, I. Effects of endogenous signals and Fusarium oxysporum on the mechanism regulating genistein synthesis and accumulation in yellow lupine and their impact on plant cell cytoskeleton. Molecules 2014, 19, 13392–13421. [Google Scholar] [CrossRef] [PubMed]
- Doke, N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol. Plant Pathol. 1983, 23, 345–357. [Google Scholar] [CrossRef]
- Stobiecki, M.; Wojtaszek, P.; Gulewicz, K. Application of solid phase extraction for profiling of quinolisidine alkaloids and phenolic compounds in Lupinus albus. Phytochem. Anal. 1997, 8, 153–158. [Google Scholar] [CrossRef]
- Morkunas, I.; Stobiecki, M.; Marczak, Ł.; Stachowiak, J.; Narożna, D.; Remlein-Starosta, D. Changes in carbohydrate and isoflavonoid metabolism in yellow lupine in response to infection by Fusarium oxysporum during the stages of seed germination and early seedling growth. Physiol. Mol. Plant Pathol. 2010, 75, 46–55. [Google Scholar] [CrossRef]
- Nichols, E.J.; Beckman, J.M.; Hadwiger, L.A. Glycosidic enzyme activity in pea tissue and pea-Fusarium solani interactions. Plant Physiol. 1980, 66, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Kozłowska, M.; Ratajczak, L.; Marczak, Ł. Role of sucrose in the development of Fusarium wilt in lupine embryo axes. Physiol. Mol. Plant Pathol. 2007, 70, 25–37. [Google Scholar] [CrossRef]









| Population and Life Parameters | Control | +GSNO | +SNP |
|---|---|---|---|
| Pre-reproductive period (days) | 6.25 ± 0.44 | 7.25 ± 0.55 | 7.1 ± 0.55 |
| Reproductive period (days) | 23.25 ± 1.0 | 12.4 ± 2.76 | 11.0 ± 2.55 |
| Post-reproductive period (days) | 1.05 ± 1.14 | 0.90 ± 0.71 | 1.1 ± 0.71 |
| Fecundity | 172.7 ± 8.62 | 82.55 ± 6.50 | 91.6 ± 5.60 |
| Longevity | 30.55 ± 1.57 | 20.55 ± 2.89 | 19.2 ± 2.64 |
| Variants | Parameter | ||||
|---|---|---|---|---|---|
| Ro | rm | λ | T | DT | |
| Control | 33.672 | 0.270 | 1.310 | 13.025 | 2.567 |
| +GSNO | 16.510 | 0.217 | 1.242 | 12.922 | 3.194 |
| +SNP | 18.322 | 0.229 | 1.257 | 12.699 | 3.027 |
| General Aspects of Aphid Probing Behavior | Control | 24 h +GSNO | 48 h +GSNO |
|---|---|---|---|
| n = 19 | n = 17 | n = 17 | |
| Total duration (h) of non-probing phase np 1 | 2.3 ± 0.5 | 2.6 ± 0.6 | 5.5 ± 1.4 |
| Total duration (h) of non-phloem probing phase C + G + F 2 | 8.4 ± 0.5 | 9.3 ± 0.8 | 10.2 ± 1.1 |
| Total duration (h) of phloem phase E1 + E2 3 | 13.2 ± 0.8 | 12.1 ± 1.1 | 8.3 ± 1.1b *,† |
| Phloem phase index 4 | 0.60± 0.03 | 0.55 ± 0.05 | 0.43 ± 0.05 *,† |
| Number of probes | 53.8 ± 9.9 | 50.6 ± 9.6 | 61.2 ± 8.7 |
| Number of probes with phloem phase | 5.3 ± 0.4 | 5.4 ± 0.5 | 4.2 ± 0.7 |
| Aphid probing behavior before 1st phloem phase | n = 19 | n = 17 | n = 15 |
| Total duration (h) of non-probing before 1st phloem phase E1 | 0.7 ±0.03 | 1.1 ± 0.4 | 0.7 ± 0.2 |
| Time to 1st phoem phase E1 | 2.1 ± 0.4 | 2.8 ± 0.8 | 6.1 ± 1.7 *,† |
| Time to 1st ingestion phase E2 | 2.1 ± 0.4 | 2.9 ± 0.8 | 6.1 ± 1.7 *,† |
| Time to 1st sustained (E2 > 10 min) ingestion phase | 2.2 ± 0.4 | 2.9 ± 0.8 | 6.4 ± 1.7 *,† |
| Aphid probing behavior related to phloem phase | n = 19 | n = 17 | n = 15 |
| Number of phloem phases E1 and E2 | 10.4 ± 1.3 | 11.4 ± 1.5 | 7.7 ± 1.4 |
| Duration (h) of 1st phloem phase E1 + E2 | 1.2 ± 0.2 | 1.7 ± 0.5 | 1.7 ± 0.6 |
| Phloem salivation index 5 | 0.02± 0.03 | 0.04 ± 0.08 | 0.05 ± 0.01 |
| General Aspects of Aphid Probing Behavior | Control | 24 h +SNP | 48 h +SNP |
|---|---|---|---|
| n = 10 | n = 12 | n = 12 | |
| Total duration (h) of non-probing phase np 1 | 9.4 ± 2.3 | 15.1 ± 2.1 | 13.6 ± 2.5 |
| Total duration (h) of non-phloem probing phase C + G + F 2 | 7.2 ± 1.3 | 4.5 ± 0.8 | 5.2 ± 1.1 |
| Total duration (h) of phloem phase E1 + E2 3 | 7.4 ± 1.9 | 4.5 ± 2.0 | 5.2 ± 1.1 |
| Phloem phase index 4 | 0.47 ± 0.07 | 0.32 ± 0.08 | 0.32 ± 0.09 |
| Number of probes | 62.6 ± 16.4 | 22.7 ± 4.0 * | 31.8 ± 6.1 |
| Number of probes with phloem phase | 4.5 ± 0.8 | 2.3 ± 0.5 * | 3.9 ± 1.0 |
| Aphid probing behavior before 1st phloem phase | n = 10 | n = 12 | n = 12 |
| Total duration (h) of non-probing before 1st phloem phase E 1 | 0.4 ± 0.2 | 1.1 ± 0.4 | 0.2 ± 0.1 |
| Time to 1st phoem phase E1 | 1.3 ± 0.3 | 4.0 ± 1.1 * | 4.2 ± 1.4 |
| Time to 1st ingestion phase E2 | 1.3 ± 0.3 | 4.1 ± 1.1 * | 4.2 ± 1.4 |
| Time to 1st sustained (E2 > 10 min) ingestion phase | 1.3 ± 0.3 | 4.7 ± 1.3 * | 5.1 ± 1.5 |
| Aphid probing behavior during phloem phase | n = 10 | n = 12 | n = 12 |
| Number of phloem phases E1 and E2 | 8.0 ± 1.6 | 4.2 ± 1.1 | 5.8 ± 1.7 |
| Duration (h) of 1st phloem phase E1 + E2 | 2.2 ± 0.7 | 1.2 ± 0.6 * | 0.9 ± 0.5 * |
| Phloem salivation index 5 | 0.06 ± 0.01 | 0.09 ± 0.03 | 0.05 ± 0.02 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźniak, A.; Formela, M.; Bilman, P.; Grześkiewicz, K.; Bednarski, W.; Marczak, Ł.; Narożna, D.; Dancewicz, K.; Mai, V.C.; Borowiak-Sobkowiak, B.; et al. The Dynamics of the Defense Strategy of Pea Induced by Exogenous Nitric Oxide in Response to Aphid Infestation. Int. J. Mol. Sci. 2017, 18, 329. https://doi.org/10.3390/ijms18020329
Woźniak A, Formela M, Bilman P, Grześkiewicz K, Bednarski W, Marczak Ł, Narożna D, Dancewicz K, Mai VC, Borowiak-Sobkowiak B, et al. The Dynamics of the Defense Strategy of Pea Induced by Exogenous Nitric Oxide in Response to Aphid Infestation. International Journal of Molecular Sciences. 2017; 18(2):329. https://doi.org/10.3390/ijms18020329
Chicago/Turabian StyleWoźniak, Agnieszka, Magda Formela, Piotr Bilman, Katarzyna Grześkiewicz, Waldemar Bednarski, Łukasz Marczak, Dorota Narożna, Katarzyna Dancewicz, Van Chung Mai, Beata Borowiak-Sobkowiak, and et al. 2017. "The Dynamics of the Defense Strategy of Pea Induced by Exogenous Nitric Oxide in Response to Aphid Infestation" International Journal of Molecular Sciences 18, no. 2: 329. https://doi.org/10.3390/ijms18020329
APA StyleWoźniak, A., Formela, M., Bilman, P., Grześkiewicz, K., Bednarski, W., Marczak, Ł., Narożna, D., Dancewicz, K., Mai, V. C., Borowiak-Sobkowiak, B., Floryszak-Wieczorek, J., Gabryś, B., & Morkunas, I. (2017). The Dynamics of the Defense Strategy of Pea Induced by Exogenous Nitric Oxide in Response to Aphid Infestation. International Journal of Molecular Sciences, 18(2), 329. https://doi.org/10.3390/ijms18020329