Role of Netrin-1 Signaling in Nerve Regeneration
Abstract
:1. Introduction
2. The Discovery of Netrin-1 and Its Immunoglobulin Superfamily Receptors
3. Crystal Structure Based Elucidation of Netrin-1 Bi-Functionality
4. Role of Netrin-1 in Spinal Cord Injury and Repair
5. Role of Netrin-1 in Optic Nerve Development and Regeneration
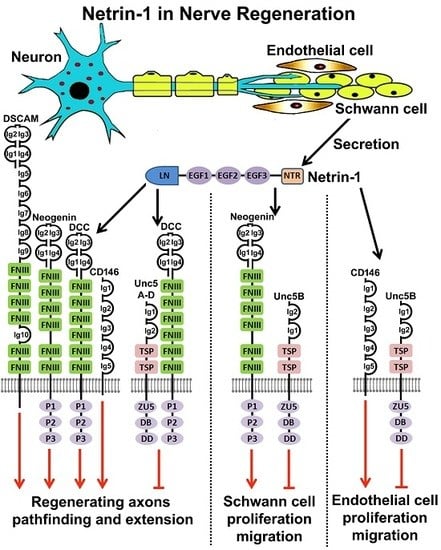
6. Netrin-1 and Netrin-1 Receptors Expression in Intact and Injured Peripheral Nerves
7. Netrin-1 Regulates Schwann Cell Proliferation and Migration
8. Netrin-1 Signaling in Peripheral Nerve Regeneration
9. Future Directions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cajal, S.R. A quelle epoque apparaissent les expansions des cellules nerveuses de la moëlle épinière du poulet? Anat. Anz. 1890, 5, 609–639. [Google Scholar]
- Cajal, S.R. La rétine des vertébrés. La Cellule 1892, 9, 121–133. [Google Scholar]
- Cajal, S.R. Textura del Sistema Nervioso del Hombre y de los Vertebrados; Moya: Madrid, Spain, 1899; Volume 2. [Google Scholar]
- Sperry, R.W. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc. Natl. Acad. Sci. USA 1963, 50, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Hedgecock, E.M.; Culotti, J.G.; Hall, D.H. The Unc-5, Unc-6, and Unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 1990, 4, 61–85. [Google Scholar] [CrossRef]
- Ishii, N.; Wadsworth, W.G.; Stern, B.D.; Culotti, J.G.; Hedgecock, E.M. Unc-6, a laminin-related protein, guides cell and pioneer axon migrations in C. elegans. Neuron 1992, 9, 873–881. [Google Scholar] [CrossRef]
- Serafini, T.; Kennedy, T.E.; Galko, M.J.; Mirzayan, C.; Jessell, T.M.; Tessier-Lavigne, M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans unc-6. Cell 1994, 78, 409–424. [Google Scholar] [CrossRef]
- Rothberg, J.M.; Hartley, D.A.; Walther, Z.; Artavanis-Tsakonas, S. Slit: An EGF-homologous locus of D. melanogaster involved in the development of the embryonic central nervous system. Cell 1988, 55, 1047–1059. [Google Scholar] [CrossRef]
- Rothberg, J.M.; Jacobs, J.R.; Goodman, C.S.; Artavanis-Tsakonas, S. Slit: An extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev. 1990, 4, 2169–2187. [Google Scholar] [CrossRef] [PubMed]
- Holzman, L.B.; Marks, R.M.; Dixit, V.M. A novel immediate-early response gene of endothelium is induced by cytokines and encodes a secreted protein. Mol. Cell. Biol. 1990, 10, 5830–5838. [Google Scholar] [CrossRef] [PubMed]
- Bartley, T.D.; Hunt, R.W.; Welcher, A.A.; Boyle, W.J.; Parker, V.P.; Lindberg, R.A.; Lu, H.S.; Colombero, A.M.; Elliott, R.L.; Guthrie, B.A.; et al. B61 is a ligand for the eck receptor protein-tyrosine kinase. Nature 1994, 368, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Kolodkin, A.L.; Matthes, D.J.; O’Connor, T.P.; Patel, N.H.; Admon, A.; Bentley, D.; Goodman, C.S. Fasciclin IV: Sequence, expression, and function during growth cone guidance in the grasshopper embryo. Neuron 1992, 9, 831–845. [Google Scholar] [CrossRef]
- Luo, Y.; Raible, D.; Raper, J.A. Collapsin: A protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell 1993, 75, 217–227. [Google Scholar] [CrossRef]
- Bashaw, G.J.; Klein, R. Signaling from axon guidance receptors. Cold Spring Harb. Perspect. Biol. 2010, 2, a001941. [Google Scholar] [CrossRef] [PubMed]
- Lai Wing Sun, K.; Correia, J.P.; Kennedy, T.E. Netrins: Versatile extracellular cues with diverse functions. Development 2011, 138, 2153–2169. [Google Scholar] [PubMed]
- Madison, R.D.; Zomorodi, A.; Robinson, G.A. Netrin-1 and peripheral nerve regeneration in the adult rat. Exp. Neurol. 2000, 161, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Manitt, C.; Colicos, M.A.; Thompson, K.M.; Rousselle, E.; Peterson, A.C.; Kennedy, T.E. Widespread expression of netrin-1 by neurons and oligodendrocytes in the adult mammalian spinal cord. J. Neurosci. 2001, 21, 3911–3922. [Google Scholar] [PubMed]
- Tanno, T.; Fujiwara, A.; Takenaka, S.; Kuwamura, M.; Tsuyama, S. Expression of a chemorepellent factor, slit2, in peripheral nerve regeneration. Biosci. Biotechnol. Biochem. 2005, 69, 2431–2434. [Google Scholar] [CrossRef] [PubMed]
- Parrinello, S.; Napoli, I.; Ribeiro, S.; Wingfield Digby, P.; Fedorova, M.; Parkinson, D.B.; Doddrell, R.D.; Nakayama, M.; Adams, R.H.; Lloyd, A.C. Ephb signaling directs peripheral nerve regeneration through sox2-dependent schwann cell sorting. Cell 2010, 143, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Pasterkamp, R.J.; Verhaagen, J. Semaphorins in axon regeneration: Developmental guidance molecules gone wrong? Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2006, 361, 1499–1511. [Google Scholar] [CrossRef] [PubMed]
- Tamariz, E.; Varela-Echavarria, A. The discovery of the growth cone and its influence on the study of axon guidance. Front. Neuroanat. 2015, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Ly, A.; Nikolaev, A.; Suresh, G.; Zheng, Y.; Tessier-Lavigne, M.; Stein, E. Dscam is a netrin receptor that collaborates with dcc in mediating turning responses to netrin-1. Cell 2008, 133, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Zhang, C.; Yan, H.; Luo, Y.; Kong, R.; Wen, P.; Ye, Z.; Chen, J.; Feng, J.; Liu, F.; et al. Cd146 acts as a novel receptor for netrin-1 in promoting angiogenesis and vascular development. Cell Res. 2015, 25, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Shipp, E.L.; Hsieh-Wilson, L.C. Profiling the sulfation specificities of glycosaminoglycan interactions with growth factors and chemotactic proteins using microarrays. Chem. Biol. 2007, 14, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Yebra, M.; Montgomery, A.M.; Diaferia, G.R.; Kaido, T.; Silletti, S.; Perez, B.; Just, M.L.; Hildbrand, S.; Hurford, R.; Florkiewicz, E.; et al. Recognition of the neural chemoattractant Netrin-1 by integrins α6β4 and α3β1 regulates epithelial cell adhesion and migration. Dev. Cell 2003, 5, 695–707. [Google Scholar] [CrossRef]
- Cirulli, V.; Yebra, M. Netrins: Beyond the brain. Nat. Rev. Mol. Cell Biol. 2007, 8, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.; Zou, Y.; Poo, M.; Tessier-Lavigne, M. Binding of dcc by Netrin-1 to mediate axon guidance independent of adenosine a2b receptor activation. Science 2001, 291, 1976–1982. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, A.G.; Davies, A.M. Chemotropic effect of specific target epithelium in the developing mammalian nervous system. Nature 1986, 323, 538–539. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, T.E.; Serafini, T.; de la Torre, J.R.; Tessier-Lavigne, M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 1994, 78, 425–435. [Google Scholar] [CrossRef]
- Van Raay, T.J.; Foskett, S.M.; Connors, T.D.; Klinger, K.W.; Landes, G.M.; Burn, T.C. The Ntn2l gene encoding a novel human netrin maps to the autosomal dominant polycystic kidney disease region on chromosome 16p13.3. Genomics 1997, 41, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Murrell, J.R.; Hunter, D.D.; Olson, P.F.; Jin, W.; Keene, D.R.; Brunken, W.J.; Burgeson, R.E. A novel member of the netrin family, β-netrin, shares homology with the β chain of laminin: Identification, expression, and functional characterization. J. Cell Biol. 2000, 151, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Yamada, K.; Sawada, M.; Nakano, S.; Mori, N.; Sawamoto, K.; Sato, K. Netrin-5 is highly expressed in neurogenic regions of the adult brain. Front. Cell. Neurosci. 2015, 9, 146. [Google Scholar] [CrossRef] [PubMed]
- Nakashiba, T.; Ikeda, T.; Nishimura, S.; Tashiro, K.; Honjo, T.; Culotti, J.G.; Itohara, S. Netrin-g1: A novel glycosyl phosphatidylinositol-linked mammalian netrin that is functionally divergent from classical netrins. J. Neurosci. 2000, 20, 6540–6550. [Google Scholar] [PubMed]
- Nakashiba, T.; Nishimura, S.; Ikeda, T.; Itohara, S. Complementary expression and neurite outgrowth activity of netrin-g subfamily members. Mech. Dev. 2002, 111, 47–60. [Google Scholar] [CrossRef]
- Meijers, R.; Puettmann-Holgado, R.; Skiniotis, G.; Liu, J.H.; Walz, T.; Wang, J.H.; Schmucker, D. Structural basis of dscam isoform specificity. Nature 2007, 449, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, X.; Zhou, X.H.; Liu, J.H.; Wu, J.; Zhang, Y.; Wang, J.H. N-terminal horseshoe conformation of dcc is functionally required for axon guidance and might be shared by other neural receptors. J. Cell Sci. 2013, 126, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.; Hinck, L.; Nishiyama, M.; Poo, M.M.; Tessier-Lavigne, M.; Stein, E. A ligand-gated association between cytoplasmic domains of Unc5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell 1999, 97, 927–941. [Google Scholar] [CrossRef]
- Chan, S.S.; Zheng, H.; Su, M.W.; Wilk, R.; Killeen, M.T.; Hedgecock, E.M.; Culotti, J.G. Unc-40, a C. elegans homolog of DCC (deleted in colorectal cancer), is required in motile cells responding to Unc-6 netrin cues. Cell 1996, 87, 187–195. [Google Scholar] [CrossRef]
- Kolodziej, P.A.; Timpe, L.C.; Mitchell, K.J.; Fried, S.R.; Goodman, C.S.; Jan, L.Y.; Jan, Y.N. Frazzled encodes a drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell 1996, 87, 197–204. [Google Scholar] [CrossRef]
- Fearon, E.R.; Cho, K.R.; Nigro, J.M.; Kern, S.E.; Simons, J.W.; Ruppert, J.M.; Hamilton, S.R.; Preisinger, A.C.; Thomas, G.; Kinzler, K.W.; et al. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science 1990, 247, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Keino-Masu, K.; Masu, M.; Hinck, L.; Leonardo, E.D.; Chan, S.S.; Culotti, J.G.; Tessier-Lavigne, M. Deleted in colorectal cancer (DCC) encodes a netrin receptor. Cell 1996, 87, 175–185. [Google Scholar] [CrossRef]
- Vielmetter, J.; Kayyem, J.F.; Roman, J.M.; Dreyer, W.J. Neogenin, an avian cell surface protein expressed during terminal neuronal differentiation, is closely related to the human tumor suppressor molecule deleted in colorectal cancer. J. Cell Biol. 1994, 127, 2009–2020. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wu, Z.; Renier, N.; Antipenko, A.; Tzvetkova-Robev, D.; Xu, Y.; Minchenko, M.; Nardi-Dei, V.; Rajashankar, K.R.; Himanen, J.; et al. Neural migration. Structures of netrin-1 bound to two receptors provide insight into its axon guidance mechanism. Science 2014, 344, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Finci, L.I.; Kruger, N.; Sun, X.; Zhang, J.; Chegkazi, M.; Wu, Y.; Schenk, G.; Mertens, H.D.; Svergun, D.I.; Zhang, Y.; et al. The crystal structure of netrin-1 in complex with DCC reveals the bifunctionality of netrin-1 as a guidance cue. Neuron 2014, 83, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Leung-Hagesteijn, C.; Spence, A.M.; Stern, B.D.; Zhou, Y.; Su, M.W.; Hedgecock, E.M.; Culotti, J.G. Unc-5, a transmembrane protein with immunoglobulin and thrombospondin type 1 domains, guides cell and pioneer axon migrations in C. elegans. Cell 1992, 71, 289–299. [Google Scholar] [CrossRef]
- Hamelin, M.; Zhou, Y.; Su, M.W.; Scott, I.M.; Culotti, J.G. Expression of the Unc-5 guidance receptor in the touch neurons of C. elegans steers their axons dorsally. Nature 1993, 364, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, E.D.; Hinck, L.; Masu, M.; Keino-Masu, K.; Ackerman, S.L.; Tessier-Lavigne, M. Vertebrate homologues of C. elegans Unc-5 are candidate netrin receptors. Nature 1997, 386, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, S.L.; Kozak, L.P.; Przyborski, S.A.; Rund, L.A.; Boyer, B.B.; Knowles, B.B. The mouse rostral cerebellar malformation gene encodes an Unc-5-like protein. Nature 1997, 386, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Engelkamp, D. Cloning of three mouse Unc5 genes and their expression patterns at mid-gestation. Mech. Dev. 2002, 118, 191–197. [Google Scholar] [CrossRef]
- Grandin, M.; Meier, M.; Delcros, J.G.; Nikodemus, D.; Reuten, R.; Patel, T.R.; Goldschneider, D.; Orriss, G.; Krahn, N.; Boussouar, A.; et al. Structural decoding of the Netrin-1/Unc5 interaction and its therapeutical implications in cancers. Cancer Cell 2016, 29, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Round, J.; Stein, E. Netrin signaling leading to directed growth cone steering. Curr. Opin. Neurobiol. 2007, 17, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, K.; Huot, Y.K.; Haendelt, M.A.; Hubert, R.; Chen, X.N.; Lyons, G.E.; Korenberg, J.R. Dscam: A novel member of the immunoglobulin superfamily maps in a down syndrome region and is involved in the development of the nervous system. Hum. Mol. Genet. 1998, 7, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, W.; Wang, L.; Kar, A.; Guan, K.L.; Rao, Y.; Wu, J.Y. Dscam functions as a Netrin receptor in commissural axon pathfinding. Proc. Natl. Acad. Sci. USA 2009, 106, 2951–2956. [Google Scholar] [CrossRef] [PubMed]
- Purohit, A.A.; Li, W.; Qu, C.; Dwyer, T.; Shao, Q.; Guan, K.L.; Liu, G. Down syndrome cell adhesion molecule (DSCAM) associates with uncoordinated-5c (Unc5c) in Netrin-1-mediated growth cone collapse. J. Biol. Chem. 2012, 287, 27126–27138. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.M.; Holzmann, B.; Breitbart, E.W.; Schmiegelow, P.; Riethmuller, G.; Johnson, J.P. Discrimination between benign and malignant cells of melanocytic lineage by two novel antigens, a glycoprotein with a molecular weight of 113,000 and a protein with a molecular weight of 76,000. Cancer Res. 1987, 47, 841–845. [Google Scholar] [PubMed]
- Wang, Y.; Teng, H.L.; Huang, Z.H. Repulsive migration of schwann cells induced by slit-2 through Ca2+-dependent Rhoa-myosin signaling. Glia 2013, 61, 710–723. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, S.; Tsukamoto, Y.; Sasaki, F.; Miki, N.; Taira, E. Involvement of gicerin, a cell adhesion molecule, in development and regeneration of chick sciatic nerve. FEBS Lett. 2003, 554, 311–314. [Google Scholar] [CrossRef]
- Taira, E.; Kohama, K.; Tsukamoto, Y.; Okumura, S.; Miki, N. Characterization of Gicerin/MUC18/CD146 in the rat nervous system. J. Cell. Physiol. 2004, 198, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Li, B.S.; Su, Q.N.; Kiyama, H.; Miki, N.; Robinow, D.R.; Zhang, L. Expression of gicerin, a novel cell adhesion molecule, is upregulated in the astrocytes after hypoglossal nerve injury in rats. Neurosci. Lett. 1999, 260, 149–152. [Google Scholar] [CrossRef]
- Tsukamoto, Y.; Taira, E.; Miki, N.; Sasaki, F. The role of Gicerin, a novel cell adhesion molecule, in development, regeneration and neoplasia. Histol. Histopathol. 2001, 16, 563–571. [Google Scholar] [PubMed]
- Liu, Y.; Stein, E.; Oliver, T.; Li, Y.; Brunken, W.J.; Koch, M.; Tessier-Lavigne, M.; Hogan, B.L. Novel role for Netrins in regulating epithelial behavior during lung branching morphogenesis. Curr. Biol. CB 2004, 14, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, X.; Liu, G.; Xiong, W.; Wu, J.; Rao, Y. Netrin signal transduction and the guanine nucleotide exchange factor dock180 in attractive signaling. Nat. Neurosci. 2008, 11, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Killeen, M.; Steven, R.; Binns, K.L.; Culotti, J.; Pawson, T. Netrin stimulates tyrosine phosphorylation of the Unc-5 family of Netrin receptors and induces shp2 binding to the RCM cytodomain. J. Biol. Chem. 2001, 276, 40917–40925. [Google Scholar] [CrossRef] [PubMed]
- Finci, L.; Zhang, Y.; Meijers, R.; Wang, J.H. Signaling mechanism of the Netrin-1 receptor DCC in axon guidance. Prog. Biophys. Mol. Biol. 2015, 118, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Geisbrecht, B.V.; Dowd, K.A.; Barfield, R.W.; Longo, P.A.; Leahy, D.J. Netrin binds discrete subdomains of DCC and Unc5 and mediates interactions between dcc and heparin. J. Biol. Chem. 2003, 278, 32561–32568. [Google Scholar] [CrossRef] [PubMed]
- Bennett, K.L.; Bradshaw, J.; Youngman, T.; Rodgers, J.; Greenfield, B.; Aruffo, A.; Linsley, P.S. Deleted in colorectal carcinoma (DCC) binds heparin via its fifth fibronectin type III domain. J. Biol. Chem. 1997, 272, 26940–26946. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Irie, F.; Inatani, M.; Tessier-Lavigne, M.; Yamaguchi, Y. Netrin-1/DCC signaling in commissural axon guidance requires cell-autonomous expression of heparan sulfate. J. Neurosci. 2007, 27, 4342–4350. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, E.; Chedotal, A. Repulsive guidance molecule/neogenin: A novel ligand-receptor system playing multiple roles in neural development. Dev. Growth Differ. 2004, 46, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Le Noble, F.; Yuan, L.; Jiang, Q.; De Lafarge, B.; Sugiyama, D.; Breant, C.; Claes, F.; de Smet, F.; Thomas, J.L.; et al. The netrin receptor Unc5b mediates guidance events controlling morphogenesis of the vascular system. Nature 2004, 432, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Larrivee, B.; Freitas, C.; Trombe, M.; Lv, X.; Delafarge, B.; Yuan, L.; Bouvree, K.; Breant, C.; Del Toro, R.; Brechot, N.; et al. Activation of the Unc5b receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev. 2007, 21, 2433–2447. [Google Scholar] [CrossRef] [PubMed]
- Low, K.; Culbertson, M.; Bradke, F.; Tessier-Lavigne, M.; Tuszynski, M.H. Netrin-1 is a novel myelin-associated inhibitor to axon growth. J. Neurosci. 2008, 28, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Manitt, C.; Wang, D.; Kennedy, T.E.; Howland, D.R. Positioned to inhibit: Netrin-1 and Netrin receptor expression after spinal cord injury. J. Neurosci. Res. 2006, 84, 1808–1820. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Seo, I.A.; Seo, E.; Seo, S.Y.; Lee, H.J.; Park, H.T. Netrin-1 induces proliferation of Schwann cells through Unc5b receptor. Biochem. Biophys. Res. Commun. 2007, 362, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Manitt, C.; Thompson, K.M.; Kennedy, T.E. Developmental shift in expression of Netrin receptors in the rat spinal cord: Predominance of Unc-5 homologues in adulthood. J. Neurosci. Res. 2004, 77, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Park, J.I.; Seo, I.A.; Lee, H.K.; Park, H.T.; Shin, S.W.; Park, Y.M.; Ahn, K.J. Netrin inhibits regenerative axon growth of adult dorsal root ganglion neurons in vitro. J. Korean Med. Sci. 2007, 22, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.J.; Seo, I.A.; Lee, H.K.; Choi, E.J.; Seo, E.H.; Lee, H.J.; Park, H.T. Down-regulation of Unc5 homologue expression after the spinal cord injury in the adult rat. Neurosci. Lett. 2007, 419, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Wehrle, R.; Camand, E.; Chedotal, A.; Sotelo, C.; Dusart, I. Expression of netrin-1, slit-1 and slit-3 but not of slit-2 after cerebellar and spinal cord lesions. Eur. J. Neurosci. 2005, 22, 2134–2144. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Laramore, C.; Shifman, M.I. The expression of chemorepulsive guidance receptors and the regenerative abilities of spinal-projecting neurons after spinal cord injury. Neuroscience 2017, 341, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Fournier, A.E.; Strittmatter, S.M. Repulsive factors and axon regeneration in the CNS. Curr. Opin. Neurobiol. 2001, 11, 89–94. [Google Scholar] [CrossRef]
- Giger, R.J.; Hollis, E.R.; Tuszynski, M.H. Guidance molecules in axon regeneration. Cold Spring Harb. Perspect. Biol. 2010, 2, a001867. [Google Scholar] [CrossRef] [PubMed]
- Mann, F.; Harris, W.A.; Holt, C.E. New views on retinal axon development: A navigation guide. Int. J. Dev. Biol. 2004, 48, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Deiner, M.S.; Kennedy, T.E.; Fazeli, A.; Serafini, T.; Tessier-Lavigne, M.; Sretavan, D.W. Netrin-1 and DCC mediate axon guidance locally at the optic disc: Loss of function leads to optic nerve hypoplasia. Neuron 1997, 19, 575–589. [Google Scholar] [CrossRef]
- Gad, J.M.; Keeling, S.L.; Shu, T.; Richards, L.J.; Cooper, H.M. The spatial and temporal expression patterns of netrin receptors, DCC and neogenin, in the developing mouse retina. Exp. Eye Res. 2000, 70, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Shewan, D.; Dwivedy, A.; Anderson, R.; Holt, C.E. Age-related changes underlie switch in Netrin-1 responsiveness as growth cones advance along visual pathway. Nat. Neurosci. 2002, 5, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Petrausch, B.; Jung, M.; Leppert, C.A.; Stuermer, C.A. Lesion-induced regulation of Netrin receptors and modification of Netrin-1 expression in the retina of fish and grafted rats. Mol. Cell. Neurosci. 2000, 16, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Ellezam, B.; Selles-Navarro, I.; Manitt, C.; Kennedy, T.E.; McKerracher, L. Expression of Netrin-1 and its receptors DCC and Unc-5h2 after axotomy and during regeneration of adult rat retinal ganglion cells. Exp. Neurol. 2001, 168, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Wizenmann, A.; Thies, E.; Klostermann, S.; Bonhoeffer, F.; Bahr, M. Appearance of target-specific guidance information for regenerating axons after cns lesions. Neuron 1993, 11, 975–983. [Google Scholar] [CrossRef]
- King, C.E.; Wallace, A.; Rodger, J.; Bartlett, C.; Beazley, L.D.; Dunlop, S.A. Transient up-regulation of retinal EphA3 and EphA5, but not ephrin-A2, coincides with re-establishment of a topographic map during optic nerve regeneration in goldfish. Exp. Neurol. 2003, 183, 593–599. [Google Scholar] [CrossRef]
- Rodger, J.; Vitale, P.N.; Tee, L.B.; King, C.E.; Bartlett, C.A.; Fall, A.; Brennan, C.; O’Shea, J.E.; Dunlop, S.A.; Beazley, L.D. EphA/ephrin-A interactions during optic nerve regeneration: Restoration of topography and regulation of ephrin-A2 expression. Mol. Cell. Neurosci. 2004, 25, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Rodger, J.; Goto, H.; Cui, Q.; Chen, P.B.; Harvey, A.R. Camp regulates axon outgrowth and guidance during optic nerve regeneration in goldfish. Mol. Cell. Neurosci. 2005, 30, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Webber, C.A.; Christie, K.J.; Cheng, C.; Martinez, J.A.; Singh, B.; Singh, V.; Thomas, D.; Zochodne, D.W. Schwann cells direct peripheral nerve regeneration through the Netrin-1 receptors, DCC and Unc5h2. Glia 2011, 59, 1503–1517. [Google Scholar] [CrossRef] [PubMed]
- Jaminet, P.; Kohler, D.; Schaufele, M.; Rahmanian-Schwarz, A.; Lotter, O.; Fornaro, M.; Ronchi, G.; Geuna, S.; Rosenberger, P.; Schaller, H.E. Evaluating the role of Netrin-1 during the early phase of peripheral nerve regeneration using the mouse median nerve model. Restor. Neurol. Neurosci. 2013, 31, 337–345. [Google Scholar] [PubMed]
- Rosenberg, A.F.; Isaacman-Beck, J.; Franzini-Armstrong, C.; Granato, M. Schwann cells and deleted in colorectal carcinoma direct regenerating motor axons towards their original path. J. Neurosci. 2014, 34, 14668–14681. [Google Scholar] [CrossRef] [PubMed]
- Jaminet, P.; Kohler, D.; Rahmanian-Schwarz, A.; Lotter, O.; Mager, A.; Fornaro, M.; Ronchi, G.; Geuna, S.; Rosenberger, P.; Schaller, H.E. Expression patterns and functional evaluation of the Unc5b receptor during the early phase of peripheral nerve regeneration using the mouse median nerve model. Microsurgery 2013, 33, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Sun, X.; Ma, J.; Ma, X.; Zhang, Y.; Li, F.; Li, Y.; Zhao, Z. Netrin-1 induces the migration of Schwann cells via p38 MAPK and PI3K-Akt signaling pathway mediated by the Unc5b receptor. Biochem. Biophys. Res. Commun. 2015, 464, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xue, C.; Yuan, Y.; Zhang, R.; Wang, Y.; Wang, Y.; Yu, B.; Liu, J.; Ding, F.; Yang, Y.; et al. The transcriptional landscape of dorsal root ganglia after sciatic nerve transection. Sci. Rep. 2015, 5, 16888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yu, B.; Gu, Y.; Zhou, S.; Qian, T.; Wang, Y.; Ding, G.; Ding, F.; Gu, X. Fibroblast-derived tenascin-C promotes Schwann cell migration through β1-integrin dependent pathway during peripheral nerve regeneration. Glia 2016, 64, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Barrette, B.; Calvo, E.; Vallieres, N.; Lacroix, S. Transcriptional profiling of the injured sciatic nerve of mice carrying the Wld(S) mutant gene: Identification of genes involved in neuroprotection, neuroinflammation, and nerve regeneration. Brain Behav. Immun. 2010, 24, 1254–1267. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Liu, Y.; Yan, J.Y.; Wang, Y.; Yao, X.; Zhou, H.X.; Lu, L.; Kong, X.H.; Feng, S.Q. Gene expression analysis at multiple time-points identifies key genes for nerve regeneration. Muscle Nerve 2016. [Google Scholar] [CrossRef] [PubMed]
- Castets, M.; Mehlen, P. Netrin-1 role in angiogenesis: To be or not to be a pro-angiogenic factor? Cell Cycle 2010, 9, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- St Croix, B. Cd146: The unveiling of a pro-angiogenic netrin receptor. Cell Res. 2015, 25, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Serafini, T.; Colamarino, S.A.; Leonardo, E.D.; Wang, H.; Beddington, R.; Skarnes, W.C.; Tessier-Lavigne, M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 1996, 87, 1001–1014. [Google Scholar] [CrossRef]
- Watanabe, K.; Tamamaki, N.; Furuta, T.; Ackerman, S.L.; Ikenaka, K.; Ono, K. Dorsally derived netrin 1 provides an inhibitory cue and elaborates the ‘waiting period’ for primary sensory axons in the developing spinal cord. Development 2006, 133, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Watanabe, K.; Sakuma, C.; Ikenaka, K.; Ono, K.; Yaginuma, H. Netrin-1 acts as a repulsive guidance cue for sensory axonal projections toward the spinal cord. J. Neurosci. 2008, 28, 10380–10385. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.Y.; Tang, B.L. Axonal regeneration in adult CNS neurons—Signaling molecules and pathways. J. Neurochem. 2006, 96, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Li, Q.; Xu, L.; Zhang, Y.; Li, D.; Ma, J.; Mao, X. Netrin-1 overexpression in bone marrow mesenchymal stem cells promotes functional recovery in a rat model of peripheral nerve injury. J. Biomed. Res. 2015, 29, 380–389. [Google Scholar] [PubMed]
- Cattin, A.L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.; Garcia Calavia, N.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef] [PubMed]
- Torigoe, K.; Tanaka, H.F.; Takahashi, A.; Awaya, A.; Hashimoto, K. Basic behavior of migratory Schwann cells in peripheral nerve regeneration. Exp. Neurol. 1996, 137, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Dun, X.P.; Parkinson, D.B. Visualizing peripheral nerve regeneration by whole mount staining. PLoS ONE 2015, 10, e0119168. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, M.; Nambi, D.; Walheim, C.; Somerville, M.; Walker, J.; Kim, L.; Ollison, L.; Diamante, G.; Vyawahare, S.; de Bellard, M.E. Effect of NRG1, GDNF, EGF and NGF in the migration of a Schwann cell precursor line. Neurochem. Res. 2010, 35, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Afshari, F.T.; Kwok, J.C.; Fawcett, J.W. Astrocyte-produced ephrins inhibit Schwann cell migration via VAV2 signaling. J. Neurosci. 2010, 30, 4246–4255. [Google Scholar] [CrossRef] [PubMed]
- Scarlato, M.; Ara, J.; Bannerman, P.; Scherer, S.; Pleasure, D. Induction of neuropilins-1 and -2 and their ligands, sema3a, sema3f, and VEGF, during wallerian degeneration in the peripheral nervous system. Exp. Neurol. 2003, 183, 489–498. [Google Scholar] [CrossRef]
- Koeberle, P.D.; Bahr, M. Growth and guidance cues for regenerating axons: Where have they gone? J. Neurobiol. 2004, 59, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Harel, N.Y.; Strittmatter, S.M. Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat. Rev. Neurosci. 2006, 7, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Bin, J.M.; Han, D.; Lai Wing Sun, K.; Croteau, L.P.; Dumontier, E.; Cloutier, J.F.; Kania, A.; Kennedy, T.E. Complete loss of Netrin-1 results in embryonic lethality and severe axon guidance defects without increased neural cell death. Cell Rep. 2015, 12, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Navankasattusas, S.; Whitehead, K.J.; Suli, A.; Sorensen, L.K.; Lim, A.H.; Zhao, J.; Park, K.W.; Wythe, J.D.; Thomas, K.R.; Chien, C.B.; et al. The Netrin receptor Unc5b promotes angiogenesis in specific vascular beds. Development 2008, 135, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Krimpenfort, P.; Song, J.Y.; Proost, N.; Zevenhoven, J.; Jonkers, J.; Berns, A. Deleted in colorectal carcinoma suppresses metastasis in p53-deficient mammary tumours. Nature 2012, 482, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Sun, D.; Hu, J.X.; Tang, F.L.; Lee, D.H.; Wang, Y.; Hu, G.; Zhu, X.J.; Zhou, J.; Mei, L.; et al. Neogenin promotes BMP2 activation of Yap and Smad1 and enhances astrocytic differentiation in developing mouse neocortex. J. Neurosci. 2016, 36, 5833–5849. [Google Scholar] [CrossRef] [PubMed]
- Witzel, C.; Rohde, C.; Brushart, T.M. Pathway sampling by regenerating peripheral axons. J. Compar. Neurol. 2005, 485, 183–190. [Google Scholar] [CrossRef] [PubMed]
- de Ruiter, G.C.; Malessy, M.J.; Alaid, A.O.; Spinner, R.J.; Engelstad, J.K.; Sorenson, E.J.; Kaufman, K.R.; Dyck, P.J.; Windebank, A.J. Misdirection of regenerating motor axons after nerve injury and repair in the rat sciatic nerve model. Exper. Neurol. 2008, 211, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Zochodne, D.W. The challenges and beauty of peripheral nerve regrowth. J. Peripher. Nerv. Syst. 2012, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]




| Gray Matter | White Matter | Injury Response | ||
|---|---|---|---|---|
| Dorsal | Ventral | |||
| Netrin-1 | Oligodendrocyte (+++) Interneurons (++) | Oligodendrocyte (+++) Motorneurons (++) | Oligodendrocyte (+++) | Down-regulated |
| DCC | Interneurons (+) | Motorneurons (++) | Glia (+) | Down-regulated |
| Neogenin | Interneurons (+) | Motorneurons (++) | Glia (+) | Down-regulated |
| Unc5A | Interneurons (+) | Motorneurons (+++) | Glia (+) | Down-regulated |
| Unc5B | Interneurons (+++) | Motorneurons (+++) | Glia (++) | Down-regulated |
| Unc5C | Interneurons (++) | Motorneurons (+++) | Glia (+) | Down-regulated |
| Unc5D | Interneurons (+) | Motorneurons (+) | Glia (+) | Down-regulated |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dun, X.-P.; Parkinson, D.B. Role of Netrin-1 Signaling in Nerve Regeneration. Int. J. Mol. Sci. 2017, 18, 491. https://doi.org/10.3390/ijms18030491
Dun X-P, Parkinson DB. Role of Netrin-1 Signaling in Nerve Regeneration. International Journal of Molecular Sciences. 2017; 18(3):491. https://doi.org/10.3390/ijms18030491
Chicago/Turabian StyleDun, Xin-Peng, and David B. Parkinson. 2017. "Role of Netrin-1 Signaling in Nerve Regeneration" International Journal of Molecular Sciences 18, no. 3: 491. https://doi.org/10.3390/ijms18030491
APA StyleDun, X.-P., & Parkinson, D. B. (2017). Role of Netrin-1 Signaling in Nerve Regeneration. International Journal of Molecular Sciences, 18(3), 491. https://doi.org/10.3390/ijms18030491