Tentative Characterization of Polyphenolic Compounds in the Male Flowers of Phoenix dactylifera by Liquid Chromatography Coupled with Mass Spectrometry and DFT
Abstract
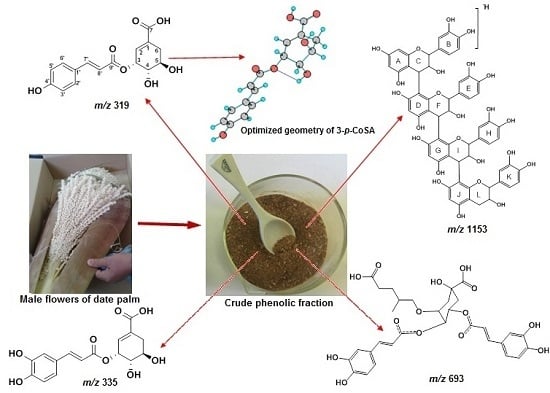
:1. Introduction
2. Results and Discussion
2.1. LC-ESI-MS and DFT Analysis of Hydroxycinnamic Acid Derivatives
2.2. LC-ESI-MS of Fatty Acids
2.3. LC-ESI-MS Analysis of Proanthocyanidins Derivatives
2.4. LC-ESI-MS Analysis of Flavonol and Flavone Derivatives
3. Material and Methods
3.1. Plant Materials
3.2. Extraction and Fractionation
3.3. High-Performance Liquid Chromatography and ESI-Mass Spectrometry of Phenolic Fraction
3.4. DFT Study
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hasan, M.; Mohieldein, A. In Vivo Evaluation of Anti Diabetic, Hypolipidemic, Antioxidative Activities of Saudi Date Seed Extract on Streptozotocin Induced Diabetic Rats. J. Clin. Diagn. Res. 2016, 10, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Coply, M.S.; Rose, P.J.; Clampham, A.; Edward, D.N.; HotRon, M.C.; Evershed, R.P. Detection of palm fruit lipids in archaeological pottery from Qasr Ibrim, Egyptian Nubia. Proc. Soc. Lond. 2001, 268, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Embarek, G.; Kokalou, E.; Kefalas, P. Phenolic profile and antioxidant activity of the Algerian ripe date palm fruit (Phoenix dactylifera). Food Chem. 2005, 89, 411–420. [Google Scholar] [CrossRef]
- Biglari, F.; Alkarkhi Abbas, F.M.; Easa, A.M. Antioxidant activity and phenolic content of various date palm (Phoenix dactylifera) fruits from Iran. Food Chem. 2008, 107, 1636–1641. [Google Scholar] [CrossRef]
- Soliman, F.A.; Soliman, A. The gonad stimulating potency of date palm male flowers. Experientia 1958, 14, 92–93. [Google Scholar] [CrossRef] [PubMed]
- Bajpayee, K.K. Ethnobotany of phoenix (Archaeae). J. Econ. Taxon. Bot. 1997, 21, 155. [Google Scholar]
- Bahmanpour, S.; Talaei, T.; Vojdani, Z.; Panjehshahin, M.R.; Poostpasand, A.; Zareei, S.; Ghaeminia, M. Effect of Phoenix dactylifera pollen on sperm parameters and reproductive system of adult male rats. Iran J. Med. Sci. 2006, 31, 208–212. [Google Scholar]
- Fawkeya, A.; Ateya, A.M. Estradiol, esteriol, estrone, and novel flavonoids from date palm pollen. Aust. J. Basic Appl. Sci. 2011, 5, 606–614. [Google Scholar]
- Jaiswal, R.; Sovdat, T.; Vivan, F.; Kuhnert, N. Profiling and characterization by LC-MSn of the chlorogenic acids and hydroxycinnamoylshikimate esters in mate (Ilex paraguariensis). J. Agric. Food Chem. 2010, 58, 5471–5484. [Google Scholar] [CrossRef] [PubMed]
- Karakose, H.; Jaiswal, R.; Kuhnert, N. Characterization and quantification of hydroxycinnamate derivatives in Stevia rebaudiana leaves by LC-MSn. J. Agric. Food Chem. 2011, 59, 10143–10150. [Google Scholar] [CrossRef] [PubMed]
- Maier, V.P.; Metzler, D.M.; Huber, A.F. 3-O-caffeoylshikimic acid (dactyliferic acid) and its isomers, a new class of enzymic browning substrates. Biochem. Biophys. Res. Commun. 1964, 14, 124. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A.; Greenham, J.; Moyna, P. Distribution of charged flavones and caffeoylshikimic acid in Palmae. Phytochemistry 1974, 13, 1557–1559. [Google Scholar] [CrossRef]
- Hamed, A.I.; Al-Ayed, A.S.; Moldoch, J.; Piacente, S.; Oleszek, W.; Stochmal, A. Profiles analysis of proanthocyanidins in the argun nut (Medemia argun—An ancient Egyptian palm) by LC-ESI-MS/MS. J. Mass Spectrom. 2014, 49, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Maldini, M.; Montora, P.; Hamed, A.I.; Mahalel, U.A.; Oleszek, W.; Piacente, S. Strong antioxidant phenolics from Acacia nilotica: Profiling by ESI-MS and quali-quantitative determination by LC-ESI-MS. J. Pharm. Biomed. Anal. 2011, 56, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Porter, J. Methods in Plant Biochemistry: 1-Plant Phenolics; Dey, P.M., Harborne, J.B., Eds.; Academic Press Inc.: San Diego, CA, USA, 1989. [Google Scholar]
- Nonaka, G.; Hasu, F.L.; Nishioka, I. Structures of dimeric, trimeric, and tetrameric procyanidins from Areca catechu L. J. Chem. Soc. Chem. Commun. 1981, 781–783. [Google Scholar] [CrossRef]
- Koolen, H.H.F.; Da Silva, F.M.A.; Gozzo, F.C.; de Souza, A.Q.L.; de Souza, A.D.L. Antioxidant, antimicrobial activities and characterization of phenolic compounds from buriti (Mauritia flexuosa) by UPLC-ESI-MS/MS. Food Res. Int. 2013, 51, 467–473. [Google Scholar] [CrossRef]
- Hamed, A.I.; Ben Said, R.; Mahalel, U.A.; Al-Ayed, A.S.; Mahmoud, A.M.; Elgebaly, H.A.; Perez, A.J.; Moldoch, J.; Stochmal, A. Fingerprinting of Strong Spermatogenesis Steroidal Saponins in Male Flowers of Phoenix dactylifera (Date Palm) by LC-ESI-MS. Nat. Prod. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Matei, M.F.; Golon, A.; Witt, M.; Kuhnert, N. Understanding the fate of chlorogenic acids in coffee roasting using mass spectrometry based targeted and non-targeted analytical strategies. Food Funct. 2012, 3, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Baderschneider, B.; Winterhalter, P. Isolation and characterization of novel benzoates, cinnamates, flavonoids and lignans from Riesling wine and screening for antioxidant activity. J. Agric. Food Agric. 2001, 49, 2788–2798. [Google Scholar] [CrossRef]
- Kuhnert, N.; Said, I.H.; Jaiswal, R. Assignment of regio- and stereochemistry of natural products using mass spectrometry chlorogenic acids and derivatives as a case study. Stud. Nat. Prod. Chem. 2014, 42, 305–339. [Google Scholar]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical composition, antioxidant properties, and thermal stability of a phytochemical enriched oil from Acai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef] [PubMed]
- Azmat, S.; Ifzal, R.; Rasheed, M.; Mohammad, F.V.; Ahmed, V.U. GC-MS analysis of N-hexane extract from seeds and leaves of Phoenix dactylifera L. J. Chem. Soc. Pak. 2010, 32, 672–676. [Google Scholar]
- Farag, M.A.; Mohsen, M.; Heinke, R.; Wessjohann, L.A. Metabolomic fingerprints of 21 date palm fruit varieties from Egypt using UPLC/PDA/ESI-Qtof-MS and MS analyzed by chemometrics. Food Res. Int. 2014, 64, 218–226. [Google Scholar] [CrossRef]
- Kolattukudy, P.E.; Walton, T.J. Structure and biosynthesis of the hydroxy fatty acids of cutin in Vicia faba leaves. Biochemistry 1972, 11, 1897–1907. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Deinzer, M.L. Tandem mass spectrometry for sequencing proanthocyanidins. Anal. Chem. 2007, 79, 1739–1748. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Kelm, A.M.; Hammerstone, F.J.; Beecher, J.; Holden, J.; Hytowitz, D.; Prior, L.R. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J. Agric. Food Chem. 2003, 51, 7513–7521. [Google Scholar] [CrossRef] [PubMed]
- Hamed, A.I.; Ben Said, R.; Kontek, B.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Stochmal, A.; Olas, B. LC-ESI-MS/MS profile of phenolic and glucosinolate compounds in Samh flour (Mesembryanthemum forsskalei Hochst) and the inhibition of oxidative stress by these compounds in human plasma. Food Res. Int. 2016, 85, 282–290. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Zakrzewski, V.G.; Montgomery, J.A.; Stratmann, R.E., Jr.; Burant, J.C.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, Y.; Wei, D.; Zhang, C.; Sun, D.; Tang, M. Direct esterification of p-nitrobenzoic acid with n-butanol using diethyl chlorophosphate in pyridine: A DFT study. Comput. Theor. Chem. 2011, 963, 13–17. [Google Scholar] [CrossRef]







| Comp. | Rt | Tentative Identification | MW | [M − H]− | Major Fragments (m/z) |
|---|---|---|---|---|---|
| Hydroxycinnamates | |||||
| 1 | 1.12 | Caffeoyl-O-hexoside | 342 | 341 | 179 (100), 161, 153, 143 |
| 2 | 16.93 | 5-O-caffeoylshikimic acid (5-CSA) | 336 | 335 | 317, 299, 291, 219, 273, 255, 179 (100), 161, 155, 137, 135, 93 |
| 3 | 17.34 | 4-O-caffeoylshikimic acid (4-CSA) | 336 | 335 | 317, 291, 219, 273, 255, 179 (100), 161 (100), 155, 137, 135, 95 |
| 4 | 18.27 | 3-O-caffeoylshikimic acid (3-CSA) | 336 | 335 | 317, 299, 291, 219, 273, 255, 179 (100), 161, 155, 137, 135, 93 |
| 5 | 21.13 | 5-O-p-coumaroylshikimic acid (5-p-CoSA) | 320 | 319 | 301, 275, 257, 243, 231, 217, 203, 171, 163 (100), 155, 145, 137, 119, 93 |
| 6 | 22.92 | 4-O-p-coumaroylshikimic acid (3-p-CoSA) | 320 | 319 | 275, 257, 199, 163 (100), 155, 145, 137, 119, 93 |
| 7 | 25.50 | caffeoyl-2-hydroxyethane-1,1,2-tricarboxylic acid | 340 | 339 | 295, 251, 179 (100) |
| 8 | 26.71 | Ferulic acid | 194 | 193 | 178, 161, 134 |
| 9 | 30.12 | caffeoylquinate shikmate derivative | 510 | 509 | 491 (100), 473, 455, 389, 371, 347, 329, 161 |
| 10 | 31.00 | 3,7-O-diferuloyl-4-O-caffeoyl quinic acid | 706 | 705 | 687, 663, 645, 559, 541, 529 (100), 527, 511, 483, 405, 337, 265, 259 |
| 11 | 32.22 | Feruloyl-O-p-coumaroyl-O-caffeoylshikimic acid | 676 | 675 | 643, 627, 603, 588, 513 (100), 495, 493, 463, 431, 375, 339, 313, 295 (100) |
| 12 | 32.64 | 5-O-p-coumaroyl-4-O-caffeoyl-4-methylpentanoic acid-5-hydroxy-3-quinate | 694 | 693 | 663, 499, 353, 345, 337, 221 |
| 13 | 32.80 | 1-O-caffeoyl-3-O-sinapoylquinic acid | 560 | 559 | 381 |
| 14 | 34.79 | Feruloyl-O-sinapoyl-O-caffeoylquinic acid | 736 | 735 | 693, 675, 559, 541, 527, 517, 513, 481, 479, 455, 323, 305 |
| 15 | 35.46 | 3-O-feruloyl-7-O-acyl-feruloyl-4-O-caffeoyl-quinic acid | 748 | 747 | 729, 705, 687, 591, 583, 574, 571 (100), 573, 559, 547, 529, 409, 381, 357, 333, 277 |
| 16 | 37.50 | (E)-3-(3,4-diacetoxy-5-methoxyphenyl)acroyl-4-O-p-coumaroyl-5-O-caffeoylquinic acid | 778 | 777 | 759, 735, 717, 693, 675, 615, 601, 559, 541, 527, 517, 513, 499, 481, 479, 455, 439, 425, 337,323, 305, 279, 271, 247, 245, 217 |
| 17 | 37.61 | Tri-caffeoylquinic acid | 712 | 711 | 679, 667, 635, 535, 387, 355, 353 |
| 18 | 40.95 | 3-O-p-coumaroyl-4-O-hydrosinapoyl-O-hexoside-5-O-caffeoylquinic acid | 870 | 869 | 707, 693 (100), 675, 661, 499, 413, 355, 337, 323, 265, 247 |
| 19 | 67.66 | p-coumaroyl-O-16-hydroxypalmatic acid | 419 | 418 | 163, 145, 119, 93 |
| Other organic acids | |||||
| 20 | 15.81 | Ascorbic acid | 176 | 175 | 157 |
| 21 | 25.51 | Trihydroxy-octadecanoic acid isomer | 330 | 329 | 314 (100), 135 |
| 22 | 29.05 | Trihydroxy-octadecadienoic acid isomer | 328 | 327 | 206 |
| 23 | 30.39 | Pentahydroxy-octadecanoic acid isomer | 348 | 347 | 329, 311, 304, 193, 161 |
| 24 | 32.42 | Trihydroxyoctadecanoic acid sulphate | 334 | 333 | 315 (100), 235, 151, 97 |
| Flavan-3-ols | |||||
| 25 | 11.63 | (E)Catechin a | 290 | 289 | 271, 247, 245 (100), 231, 221, 205, 179, 167, 151, 139, 125, 109 |
| 26 | 12.20 | (E)Catechin–(E)Catechin–(E)Catechin a | 866 | 865 | 847, 739, 713, 647, 627, 617, 577, 575 (100), 559, 515, 449, 477, 404, 327, 287 |
| 27 | 21.42 | (E)Catechin–(E)Catechin–(E)Catechin a | 866 | 865 | 847, 739, 713, 647, 627, 617, 577, 575 (100), 559, 515, 449, 477, 404, 327, 287 |
| 28 | 27.56 | (E)Catechin–(E)Gallocatechin a | 594 | 593 | 575, 557, 549, 513, 487, 473, 449, 441, 407, 343, 305 (100), 289, 187 |
| Flavan-3-ols | |||||
| 29 | 27.61 | (E)Catechin–(E)Catechin a | 578 | 577 | 435, 425, 407, 289 (100), 287 |
| 30 | 30.54 | (E)Catechin–(E)Catechin–(E)Catechin–(E)Catechina | 1154 | 1153 | 1065, 1043, 1027 (100), 983, 947, 865 (100), 711, 701, 693, 651, 605, 575, 549, 425, 407, 289 |
| Anthocyanidins | |||||
| 31 | 25.19 | Cyanidin | 287 | 286 | 271, 225, 151 (100), 135, 125, 107 |
| 32 | 30.27 | Pelargonidin dimethyl derivative | 301 | 300 | 271 (100), 243, 163 |
| Flavonols and flavones | |||||
| 33 | 21.23 | Dihexosylisorhamentin | 640 | 639 | 611, 477, 315 (100), 300, 280, 270, 255, 242, 214, 205 |
| 34 | 21.53 | Hexosylquercetin | 464 | 463 | 343, 301(100), 217 |
| 35 | 21.83 | Dihexosylquercetin | 626 | 625 | 463, 314, 301 (100) |
| 36 | 23.54 | Gliricidin or 3-O-methylorobol | 300 | 299 | 284 (100), 272, 271, 255, 254 |
| 37 | 24.21 | Rhamnosylhexosyl luteolin | 594 | 593 | 575, 447 (100), 429, 327, 285 (100), 257, 241, 215, 195, 177 |
| 38 | 24.47 | Isorhamentin b | 315 | 314 | 299 (100), 286, 285, 271, 255, 243, 230 |
| 39 | 24.67 | Rhamnosylhexosyl methyl quercetin | 624 | 623 | 608, 591, 519, 477, 459, 339, 315 (100), 299 |
| 40 | 25.14 | Dihydrokaempferol | 288 | 287 | 272, 225, 160, 151, 139, 125, 107 |
| 41 | 25.88 | Isorhamentin hexoside | 478 | 477 | 409, 357, 329, 315 (100), 300, 271, 243, 173 |
| 42 | 26.65 | Kaempferol | 286 | 285 | 257, 241, 217,213, 197 |
| 43 | 26.76 | Isorhamentin b | 315 | 314 | 299, 286 (100), 285, 271 |
| 44 | 26.85 | Orientin or Isoorientin | 448 | 447 | 299 (100), 284 |
| 45 | 28.22 | (Orientin or Isoorientin)-sulphate | 528 | 527 | 447, 299 |
| 46 | 28.90 | Quercetin rutinoside | 610 | 609 | 591, 465, 463, 373, 343, 301 (100), 287, 271, 255, 226, 179 |
| 47 | 29.73 | Gliricidin or 3-O-methylorobol | 300 | 299 | 284 (100), 272, 271, 255, 254, 202 |
| 48 | 29.99 | quercetin acetyl-hexoside | 506 | 505 | 463, 301 (100), 300 |
| 49 | 31.54 | Hexosyl-acyl-isorhamnetin | 520 | 519 | 459, 451, 357, 315 (100), 314, 299, 285, 243, 204, 187, 151 |
| 50 | 31.64 | Chrysoeriol hexoside | 462 | 461 | 446, 299 |
| 51 | 34.08 | Rhamnosyl-hexosyl-acyl-quercetin | 650 | 649 | 607, 589, 503, 485, 407, 341, 299 (100), 284 |
| 52 | 34.90 | Rhamnosylhexosyl-methyl-quercetin | 612 | 611 | 594, 521, 491, 449, 327, 286 |
| 53 | 35.84 | Quercetin | 302 | 301 | 273, 257, 229, 215 (100), 211, 193, 179, 169, 151, 111, 107 |
| Compound | ΔE | ΔE + ZPE | ΔH | ΔG | Population (%) |
|---|---|---|---|---|---|
| 3-CSA | 0.0 | 0.0 | 0.0 | 0.0 | 67 |
| 4-CSA | 0.3 | 0.4 | 0.2 | 1.5 | 5 |
| 5-CSA | 0.1 | 0.0 | 0.1 | 0.5 | 28 |
| 3-p-CoSA | 0.0 | 0.0 | 0.0 | 0.0 | 76 |
| 4-p-CoSA | 0.3 | 0.5 | 0.3 | 1.5 | 9 |
| 5-p-CoSA | 0.0 | 0.2 | 0.1 | 0.9 | 15 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Said, R.; Hamed, A.I.; Mahalel, U.A.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative Characterization of Polyphenolic Compounds in the Male Flowers of Phoenix dactylifera by Liquid Chromatography Coupled with Mass Spectrometry and DFT. Int. J. Mol. Sci. 2017, 18, 512. https://doi.org/10.3390/ijms18030512
Ben Said R, Hamed AI, Mahalel UA, Al-Ayed AS, Kowalczyk M, Moldoch J, Oleszek W, Stochmal A. Tentative Characterization of Polyphenolic Compounds in the Male Flowers of Phoenix dactylifera by Liquid Chromatography Coupled with Mass Spectrometry and DFT. International Journal of Molecular Sciences. 2017; 18(3):512. https://doi.org/10.3390/ijms18030512
Chicago/Turabian StyleBen Said, Ridha, Arafa I. Hamed, Usam A. Mahalel, Abdullah Sulaiman Al-Ayed, Mariusz Kowalczyk, Jaroslaw Moldoch, Wieslaw Oleszek, and Anna Stochmal. 2017. "Tentative Characterization of Polyphenolic Compounds in the Male Flowers of Phoenix dactylifera by Liquid Chromatography Coupled with Mass Spectrometry and DFT" International Journal of Molecular Sciences 18, no. 3: 512. https://doi.org/10.3390/ijms18030512
APA StyleBen Said, R., Hamed, A. I., Mahalel, U. A., Al-Ayed, A. S., Kowalczyk, M., Moldoch, J., Oleszek, W., & Stochmal, A. (2017). Tentative Characterization of Polyphenolic Compounds in the Male Flowers of Phoenix dactylifera by Liquid Chromatography Coupled with Mass Spectrometry and DFT. International Journal of Molecular Sciences, 18(3), 512. https://doi.org/10.3390/ijms18030512