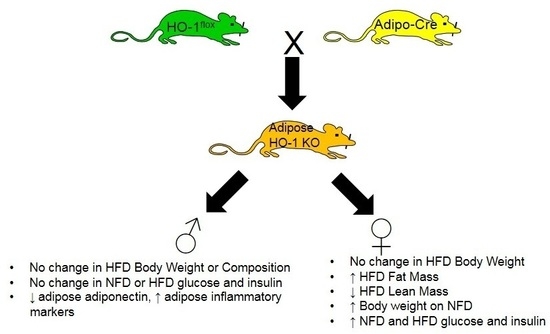
Sex-Dependent Effects of HO-1 Deletion from Adipocytes in Mice
Abstract
:1. Introduction
2. Results
2.1. Adipocyte-Specific Knockout of HO-1 Has No Effect on Body Weight, but Alters Body Composition in Female Knockout Mice Fed a High-Fat Diet
2.2. Adipocyte-Specific HO-1 Knockout Results in Sustained Increases in Fasting Blood Glucose Levels and Hyperinsulinemia in Female but Not Male Mice
2.3. Adipocyte-Specific HO-1 Knockout Mice Exhibit Alterations in Adipose HO-1 Activity without Any Changes in Kidney or Liver HO-1 Activity and Express dsRed Protein Following Cre-Mediated Deletion of the HO-1 Allele
2.4. Loss of HO-1 Decreases Adiponectin, PGC1α, and SIRT1 and Increases Markers of Inflammation in Adipose
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Body Composition (EchoMRI)
4.3. Fasting Glucose and Insulin
4.4. Heme Oxygenase Assay
4.5. Quantitative Real-Time PCR Analysis
4.6. Western Blot Analysis
4.7. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Collaboration, N.C.D.R.F. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar]
- Mathieu, P.; Lemieux, I.; Despres, J.P. Obesity, inflammation, and cardiovascular risk. Clin. Pharmacol. Ther. 2010, 87, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Lazo, M.; Hernaez, R.; Eberhardt, M.S.; Bonekamp, S.; Kamel, I.; Guallar, E.; Koteish, A.; Brancati, F.L.; Clark, J.M. Prevalence of nonalcoholic fatty liver disease in the united states: The third national health and nutrition examination survey, 1988–1994. Am. J. Epidemiol. 2013, 178, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, H.C.; Chalasani, N. Nonalcoholic fatty liver disease: An emerging threat to obese and diabetic individuals. Ann. N. Y. Acad. Sci. 2013, 1281, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.G.; Kappas, A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008, 60, 79–127. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.J.; Drummond, G.; Kim, D.H.; Li, M.; Kruger, A.L.; Ikehara, S.; Abraham, N.G. L-4f treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice. J. Lipid Res. 2008, 49, 1658–1669. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Peterson, S.; Husney, D.; Inaba, M.; Guo, K.; Kappas, A.; Ikehara, S.; Abraham, N.G. Long-lasting expression of HO-1 delays progression of type I diabetes in nod mice. Cell Cycle 2007, 6, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kim, D.H.; Tsenovoy, P.L.; Peterson, S.J.; Rezzani, R.; Rodella, L.F.; Aronow, W.S.; Ikehara, S.; Abraham, N.G. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes 2008, 57, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.; Li, M.; Vanella, L.; Kim, D.H.; Rezzani, R.; Rodella, L.; Sodhi, K.; Canestraro, M.; Martasek, P.; Peterson, S.J.; et al. Adipocyte heme oxygenase-1 induction attenuates metabolic syndrome in both male and female obese mice. Hypertension 2010, 56, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Ndisang, J.F.; Jadhav, A. Up-regulating the hemeoxygenase system enhances insulin sensitivity and improves glucose metabolism in insulin-resistant diabetes in goto-kakizaki rats. Endocrinology 2009, 150, 2627–2636. [Google Scholar] [CrossRef] [PubMed]
- Ndisang, J.F.; Lane, N.; Syed, N.; Jadhav, A. Up-regulating the heme oxygenase system with hemin improves insulin sensitivity and glucose metabolism in adult spontaneously hypertensive rats. Endocrinology 2010, 151, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Csongradi, E.; Docarmo, J.M.; Dubinion, J.H.; Vera, T.; Stec, D.E. Chronic HO-1 induction with cobalt protoporphyrin (copp) treatment increases oxygen consumption, activity, heat production and lowers body weight in obese melanocortin-4 receptor-deficient mice. Int. J. Obes. 2012, 36, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Chiang, M.T.; Chau, L.Y. Adipose overexpression of heme oxygenase-1 does not protect against high fat diet-induced insulin resistance in mice. PLoS ONE 2013, 8, e55369. [Google Scholar] [CrossRef] [PubMed]
- Ndisang, J.F.; Lane, N.; Jadhav, A. The heme oxygenase system abates hyperglycemia in zucker diabetic fatty rats by potentiating insulin-sensitizing pathways. Endocrinology 2009, 150, 2098–2108. [Google Scholar] [CrossRef] [PubMed]
- Vanella, L.; Li Volti, G.; Guccione, S.; Rappazzo, G.; Salvo, E.; Pappalardo, M.; Forte, S.; Schwartzman, M.L.; Abraham, N.G. Heme oxygenase-2/adiponectin protein-protein interaction in metabolic syndrome. Biochem. Biophys. Res. Commun. 2013, 432, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.H.; Scherer, P.E. Adipose tissue, inflammation, and cardiovascular disease. Circ. Res. 2005, 96, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.K.; Ciaraldi, T.; Henry, R.R. Adiponectin in health and disease. Diabetes Obes. Metab. 2007, 9, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Shibata, R.; Ouchi, N.; Murohara, T. Adiponectin and cardiovascular disease. Circ. J. 2009, 73, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Kruger, A.L.; Peterson, S.; Turkseven, S.; Kaminski, P.M.; Zhang, F.F.; Quan, S.; Wolin, M.S.; Abraham, N.G. D-4f induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation 2005, 111, 3126–3134. [Google Scholar] [CrossRef] [PubMed]
- Issan, Y.; Hochhauser, E.; Kornowski, R.; Leshem-Lev, D.; Lev, E.; Sharoni, R.; Vanella, L.; Puri, N.; Laniado-Schwartzman, M.; Abraham, N.G.; et al. Endothelial progenitor cell function inversely correlates with long-term glucose control in diabetic patients: Association with the attenuation of the heme oxygenase-adiponectin axis. Can. J. Cardiol. 2012, 28, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, J.; Wang, X.; Yu, S.; Kershaw, E.E.; Chiu, P.C.; Dushay, J.; Estall, J.L.; Klein, U.; Maratos-Flier, E.; Rosen, E.D. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 2011, 13, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Mamiya, T.; Katsuoka, F.; Hirayama, A.; Nakajima, O.; Kobayashi, A.; Maher, J.M.; Matsui, H.; Hyodo, I.; Yamamoto, M.; Hosoya, T. Hepatocyte-specific deletion of heme oxygenase-1 disrupts redox homeostasis in basal and oxidative environments. Tohoku J. Exp. Med. 2008, 216, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Hoyenga, K.B.; Hoyenga, K.T. Gender and energy balance: Sex differences in adaptations for feast and famine. Physiol. Behav. 1982, 28, 545–563. [Google Scholar] [CrossRef]
- Shi, H.; Clegg, D.J. Sex differences in the regulation of body weight. Physiol. Behav. 2009, 97, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C.; Sainsbury, A.; The Stock Conference 2008 Working Group. Sex differences in obesity and the regulation of energy homeostasis. Obes. Rev. 2009, 10, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F.; Roman, R.J. Androgens and hypertension: Role in both males and females? Hypertension 2011, 57, 681–682. [Google Scholar] [CrossRef] [PubMed]
- Maranon, R.; Reckelhoff, J.F. Sex and gender differences in control of blood pressure. Clin. Sci. 2013, 125, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Toth, B.; Yokoyama, Y.; Kuebler, J.F.; Schwacha, M.G.; Rue, L.W., 3rd; Bland, K.I.; Chaudry, I.H. Sex differences in hepatic heme oxygenase expression and activity following trauma and hemorrhagic shock. Arch. Surg. 2003, 138, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Bonacasa, B.; Perez, C.; Salom, M.G.; Lopez, B.; Saez-Belmonte, F.; Martinez, P.; Casas, T.; Fenoy, F.J.; Rodriguez, F. Sexual dimorphism in renal heme-heme oxygenase system in the streptozotocin diabetic rats. Curr. Pharm. Des. 2013, 19, 2678–2686. [Google Scholar] [CrossRef]
- Posa, A.; Kupai, K.; Menesi, R.; Szalai, Z.; Szabo, R.; Pinter, Z.; Palfi, G.; Gyongyosi, M.; Berko, A.; Pavo, I.; et al. Sexual dimorphism of cardiovascular ischemia susceptibility is mediated by heme oxygenase. Oxid. Med. Cell. Longev. 2013, 2013, 521563. [Google Scholar] [CrossRef]
- Manneras-Holm, L.; Benrick, A.; Stener-Victorin, E. Gene expression in subcutaneous adipose tissue differs in women with polycystic ovary syndrome and controls matched pair-wise for age, body weight, and body mass index. Adipocyte 2014, 3, 190–196. [Google Scholar] [CrossRef]
- Kim, D.H.; Burgess, A.P.; Li, M.; Tsenovoy, P.L.; Addabbo, F.; McClung, J.A.; Puri, N.; Abraham, N.G. Heme oxygenase-mediated increases in adiponectin decrease fat content and inflammatory cytokines tumor necrosis factor-α and interleukin-6 in zucker rats and reduce adipogenesis in human mesenchymal stem cells. J. Pharmacol. Exp. Ther. 2008, 325, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Ndisang, J.F. Role of the heme oxygenase-adiponectin-atrial natriuretic peptide axis in renal function. Curr. Pharm. Des. 2015, 21, 4380–4391. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Kimura, M.; Ng, C.; He, J.; Keshvari, S.; Rose, F.J.; Barclay, J.L.; Whitehead, J.P. Induction of heme-oxygenase-1 (HO-1) does not enhance adiponectin production in human adipocytes: Evidence against a direct HO-1-adiponectin axis. Mol. Cell. Endocrinol. 2015, 413, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Metoyer, C.F.; Pruitt, K. The role of sirtuin proteins in obesity. Pathophysiology 2008, 15, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Nogueiras, R.; Habegger, K.M.; Chaudhary, N.; Finan, B.; Banks, A.S.; Dietrich, M.O.; Horvath, T.L.; Sinclair, D.A.; Pfluger, P.T.; Tschop, M.H. Sirtuin 1 and sirtuin 3: Physiological modulators of metabolism. Physiol. Rev. 2012, 92, 1479–1514. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, Y.; Li, M.; Geng, C.; Xu, H.; Yang, Y.; Guo, Y.; Jiao, T.; Fang, F.; Chang, Y. Sirt1 mediates the effect of the heme oxygenase inducer, cobalt protoporphyrin, on ameliorating liver metabolic damage caused by a high-fat diet. J. Hepatol. 2015, 63, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, K.; Puri, N.; Favero, G.; Stevens, S.; Meadows, C.; Abraham, N.G.; Rezzani, R.; Ansinelli, H.; Lebovics, E.; Shapiro, J.I. Fructose mediated non-alcoholic fatty liver is attenuated by HO-1-SIRT1 module in murine hepatocytes and mice fed a high fructose diet. PLoS ONE 2015, 10, e0128648. [Google Scholar] [CrossRef] [PubMed]
- Willis, D.; Moore, A.R.; Frederick, R.; Willoughby, D.A. Heme oxygenase: A novel target for the modulation of the inflammatory response. Nat. Med. 1996, 2, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Kushida, T.; Li, V.G.; Quan, S.; Goodman, A.; Abraham, N.G. Role of human heme oxygenase-1 in attenuating TNF-α-mediated inflammation injury in endothelial cells. J. Cell. Biochem. 2002, 87, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Sawle, P.; Foresti, R.; Mann, B.E.; Johnson, T.R.; Green, C.J.; Motterlini, R. Carbon monoxide-releasing molecules (co-RMS) attenuate the inflammatory response elicited by lipopolysaccharide in raw264.7 murine macrophages. Br. J. Pharmacol. 2005, 145, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Vogel, M.E.; Zucker, S.D. Bilirubin acts as an endogenous regulator of inflammation by disrupting adhesion molecule-mediated leukocyte migration. Inflamm. Cell Signal. 2016, 3. [Google Scholar] [CrossRef]
- Hosick, P.A.; AlAmodi, A.A.; Storm, M.V.; Gousset, M.U.; Pruett, B.E.; Gray, W., 3rd; Stout, J.; Stec, D.E. Chronic carbon monoxide treatment attenuates development of obesity and remodels adipocytes in mice fed a high-fat diet. Int. J. Obes. (Lond) 2014, 38, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Vera, T.; Kelsen, S.; Yanes, L.L.; Reckelhoff, J.F.; Stec, D.E. HO-1 induction lowers blood pressure and superoxide production in the renal medulla of angiotensin ii hypertensive mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1472–R1478. [Google Scholar] [CrossRef] [PubMed]
- Vera, T.; Kelsen, S.; Stec, D.E. Kidney-specific induction of heme oxygenase-1 prevents angiotensin II hypertension. Hypertension 2008, 52, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Csongradi, E.; Storm, M.V.; Stec, D.E. Renal inhibition of heme oxygenase-1 increases blood pressure in angiotensin II-dependent hypertension. Int. J. Hypertens. 2012, 2012, 497213. [Google Scholar] [CrossRef] [PubMed]








| Parameter | Sex | Flox | KO | p |
|---|---|---|---|---|
| Body Weight (grams) | Males | 31 ± 1 (n = 9) | 31 ± 0.5 (n = 11) | N.S. |
| Body Weight (grams) | Females | 22 ± 0.6 (n = 6) | 25 ± 0.6 (n = 6) | 0.03 |
| Fasting blood glucose (mg/dL) | Males | 114 ± 5 (n = 9) | 118 ± 5 (n = 11) | N.S. |
| Fasting blood insulin (ng/mL) | Males | 0.7 ± 0.12 (n = 6) | 0.91 ± 0.29 (n = 6) | N.S. |
| Fasting blood glucose (mg/dL) | Females | 99 ± 5 (n = 6) | 119 ± 5 (n = 6) | 0.01 |
| Fasting blood insulin (ng/mL) | Females | 0.59 ± 0.03 (n = 6) | 0.82 ± 0.05 (n = 6) | 0.007 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosick, P.A.; Weeks, M.F.; Hankins, M.W.; Moore, K.H.; Stec, D.E. Sex-Dependent Effects of HO-1 Deletion from Adipocytes in Mice. Int. J. Mol. Sci. 2017, 18, 611. https://doi.org/10.3390/ijms18030611
Hosick PA, Weeks MF, Hankins MW, Moore KH, Stec DE. Sex-Dependent Effects of HO-1 Deletion from Adipocytes in Mice. International Journal of Molecular Sciences. 2017; 18(3):611. https://doi.org/10.3390/ijms18030611
Chicago/Turabian StyleHosick, Peter A., Mary Frances Weeks, Michael W. Hankins, Kyle H. Moore, and David E. Stec. 2017. "Sex-Dependent Effects of HO-1 Deletion from Adipocytes in Mice" International Journal of Molecular Sciences 18, no. 3: 611. https://doi.org/10.3390/ijms18030611
APA StyleHosick, P. A., Weeks, M. F., Hankins, M. W., Moore, K. H., & Stec, D. E. (2017). Sex-Dependent Effects of HO-1 Deletion from Adipocytes in Mice. International Journal of Molecular Sciences, 18(3), 611. https://doi.org/10.3390/ijms18030611