MMP-2 and 9 in Chronic Kidney Disease
Abstract
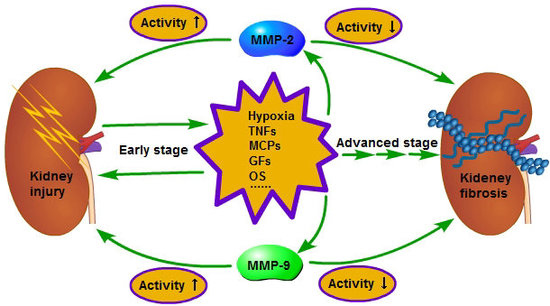
:1. Introduction
2. The Regulation of Matrix Metalloproteinase-2 (MMP-2) and Matrix Metalloproteinase-9 (MMP-9) Expression
3. The Regulation of MMP-2 and MMP-9 Activity
3.1. The Basic Pathway of the Activity Regulation of MMP-2 and MMP-9
3.2. Effects of Cell Membrane Structural Change on MMP-2 and MMP-9 Activity
4. Role of MMP-2 and MMP-9 in Chronic Kidney Disease (CKD)
4.1. The Interaction between MMP-2, 9 and Tumor Necrosis Factors (TNFs) Promotes CKD Progression
4.2. The Effects of Interaction between MMP-2, 9 and Monocyte Chemoattractant Proteins (MCPs) on CKD
4.3. The Effects of Interaction between MMP-2, 9 and Growth Factors (GFs) on CKD
4.4. The Effects of Interaction between MMP-2, 9 and Oxidative Stress on CKD
5. The Possible Relationship between MMP-2, 9 and Their Activating Molecule MMP-14 and Their Non-Proteolytic Functions with CKD
5.1. The Possible Relationship between Non-Proteolytic Functions of MMP-2 and CKD
5.2. The Possible Relationship between Non-Proteolytic Functions of MMP-9 and CKD
5.3. The Possible Relationship between Non-Proteolytic Functions of MMP-14 and CKD
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Romanowski, K.; Clark, E.G.; Levin, A.; Cook, V.J.; Johnston, J.C. Tuberculosis and chronic kidney disease: An emerging global syndemic. Kidney Int. 2016, 90, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Nugent, R.A.; Fathima, S.F.; Feigl, A.B.; Chyung, D. The burden of chronic kidney disease on developing nations: A 21st century challenge in global health. Nephron. Clin. Pract. 2011, 118, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef]
- Meran, S.; Steadman, R. Fibroblasts and myofibroblasts in renal fibrosis. Int. J. Exp. Pathol. 2011, 92, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Pei, G.; Zeng, R.; Han, M.; Liao, P.; Zhou, X.; Li, Y. Renal interstitial infiltration and tertiary lymphoid organ neogenesis in IgA nephropathy. Clin. J. Am. Soc. Nephrol. 2014, 9, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Vielhauer, V.; Mayadas, T.N. Functions of TNF and its receptors in renal disease: Distinct roles in inflammatory tissue injury and immune regulation. Semin. Nephrol. 2007, 27, 286–308. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.; Mouttalib, S.; Delage, C.; Calise, D.; Maoret, J.J.; Pradère, J.P. Dual effect of chemokine CCL7/MCP-3 in the development of renal tubulointerstitial fibrosis. Biochem. Biophys. Res. Commun. 2013, 438, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Boor, P.; Floege, J. Chronic kidney disease growth factors in renal fibrosis. Clin. Exp. Pharmacol. Physiol. 2011, 38, 441–500. [Google Scholar] [CrossRef] [PubMed]
- Wiercinska, E.; Naber, H.P.H.; Pardali, E.; Pluijm, G.V.D.; Dam, H.V.; Dijke, P.T. The TGF-β/Smad pathway induces breast cancer cell invasion through the up-regulation of matrix metalloproteinase 2 and 9 in a spheroid invasion model system. Breast Cancer Res. Treat. 2011, 128, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tan, J.; Zhang, Y.; Han, N.; Di, X.; Xiao, T. DLK1 promotes lung cancer cell invasion through upregulation of MMP-9 expression depending on Notch signaling. PLoS ONE 2014, 9, e91509. [Google Scholar]
- Gross, J.; Lapiere, C.M. Collagenolytic activity in amphibian tissue. A tissue culture assay. Proc. Natl. Acad. Sci. USA 1962, 48, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef] [PubMed]
- García-Pardo, A.; Opdenakker, G. Nonproteolytic functions of matrix metalloproteinases in pathology and insights for the development of novel therapeutic inhibitors. Metalloprotein. Med. 2015, 2, 19–28. [Google Scholar] [CrossRef]
- Piccard, H.; Pe, V.D.S.; Opdenakker, G. Hemopexin domains as multifunctional liganding modules in matrix metalloproteinases and other proteins. J. Leukocyte Biol. 2007, 81, 870–893. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhang, J.; Cai, D.H.; Zeng, L. Effect of high glucose exposure on connective tissue growth factor expression in cultured human renal tubular epithelial cells and the role of p38MAPK pathway. J. Southern Med. Univ. 2009, 29, 50–53. [Google Scholar]
- Yong, H.Y.; Kim, I.Y.; Kim, J.S.; Moon, A. ErbB2-enhanced invasiveness of H-Ras MCF10A breast cells requires MMP-13 and uPA upregulation via p38 MAPK signaling. Int. J. Oncol. 2010, 36, 501–507. [Google Scholar] [PubMed]
- Jing, S.W.; Wang, Y.D.; Kuroda, M.; Su, J.W.; Sun, G.G.; Liu, Q. HIF-1α contributes to hypoxia-induced invasion and metastasis of esophageal carcinoma via inhibiting E-cadherin and promoting MMP-2 expression. Acta Med. Okayama 2012, 66, 399–407. [Google Scholar] [PubMed]
- Zhang, G.; Kernan, K.A.; Collins, S.J.; Cai, X.; Lópezguisa, J.M.; Degen, J.L. Plasmin (ogen) promotes renal interstitial fibrosis by promoting epithelial-to-mesenchymal transition: Role of plasmin-activated signals. J. Am. Soc. Nephrol. 2007, 18, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Monea, S.; Lehti, K.; Keski-Oja, J.; Mignatti, P. Plasmin activates pro-matrix metalloproteinase-2 with a membrane-type 1 matrix metalloproteinase-dependent mechanism. J. Cell. Physiol. 2002, 192, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, K.; Ulazka, B.; Mysliwiec, M.; Pawlak, D. Vascular endothelial growth factor and uPA/suPAR system in early and advanced chronic kidney disease patients: A new link between angiogenesis and hyperfibrinolysis? Transl. Res. 2012, 160, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Siconolfi, L.B.; Seeds, N.W. Mice lacking tissue plasminogen activator and urokinase plasminogen activator genes show attenuated matrix metalloproteases activity after sciatic nerve crush. J. Neurosci. Res. 2003, 74, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.M.; Fridman, R. TIMP-2 (tissue inhibitor of metalloproteinase-2) regulates MMP-2 (matrix metalloproteinase-2) activity in the extracellular environment after pro-MMP-2 activation by MT-1 (membrane type 1)-MMP. Biochem. J. 2003, 374, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.; Chvyrkova, I.; Bernardo, M.M.; Hernandez-Barrantes, S.; Fridman, R. Pro-MMP-9 activation by the MT1-MMP/MMP-2 axis and MMP-3: Role of TIMP-2 and plasma membranes. Biochem. Biophys. Res. Commun. 2003, 308, 386–395. [Google Scholar] [CrossRef]
- Ala-Aho, R.; Kähäri, V.M. Collagenases in cancer. Biochimie 2005, 87, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, R.; Zhao, S.; Liu, L.; Guo, S.; Wu, N. Reactive oxygen species released from hypoxic hepatocytes regulates MMP-2 expression in hepatic stellate cells. Int. J. Mol. Sci. 2011, 12, 2434–2447. [Google Scholar] [CrossRef] [PubMed]
- Hyttinen, J.M.; Niittykoski, M.; Salminen, A.; Kaarniranta, K. Maturation of autophagosomes and endosomes: A key role for Rab7. Biochim. Biophys. Acta 2013, 1833, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Kukulski, W.; Schorb, M.; Kaksonen, M.; Briggs, J.A. Plasma membrane reshaping during endocytosis is revealed by time-resolved electron tomography. Cell 2012, 150, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Laura, A.; Dada Lynn, C.; Welch Guo, F.Z.; Ronen, B.S.; Aaron Ciechanover.; Jacob, I. Phosphorylation and ubiquitination are necessary for Na, K-ATPase endocytosis during hypoxia. Cell Signal. 2007, 19, 1893–1898. [Google Scholar]
- Gusarova, G.A.; Dada, L.A.; Kelly, A.M.; Brodie, C.; Witters, L.A.; Chandel, N.S. α1-AMP-activated protein kinase regulates hypoxia-induced Na, K-ATPase endocytosis via direct phosphorylation of protein kinase Cζ. Mol. Cell. Biol. 2009, 29, 3455–3464. [Google Scholar] [CrossRef] [PubMed]
- Chow, A.K.; Cena, J.; Elyazbi, A.F.; Crawford, B.D.; Holt, A.; Cho, W.J. Caveolin-1 inhibits matrix metalloproteinase-2 activity in the heart. J. Mol. Cell. Cardiol. 2007, 42, 896–901. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, S.J.; English, J.L.; Holway, N.; Murphy, G. Cellular cholesterol regulates MT1 MMP dependent activation of MMP 2 via MEK-1 in HT1080 fibrosarcoma cells. FEBS Lett. 2004, 566, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.Y.; Zhi, W.; Yi, P.L. Endocytosis mediated by Caveolin-1 inhibits activity of matrix metalloproteinase-2 in human renal proximal tubular cells under hypoxia. Int. J. Clin. Exp. Pathol. 2016, 9, 1276–1284. [Google Scholar]
- Albrechtsen, R.; Kveiborg, M.; Stautz, D.; Vikeså, J.; Noer, J.B.; Kotzsh, A. ADAM12 redistributes and activates MMP-14, resulting in gelatin degradation, reduced apoptosis and increased tumor growth. J. Cell Sci. 2013, 126, 4707–4720. [Google Scholar] [CrossRef] [PubMed]
- Labrecque, L.; Nyalendo, C.; Langlois, S.; Durocher, Y.; Roghi, C.; Murphy, G. Src-mediated tyrosine phosphorylation of caveolin-1 induces its association with membrane type 1 matrix metalloproteinase. J. Biol. Chem. 2004, 279, 52132–52140. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Chung, H.S. Caveolin-1 inhibits membrane-type 1 matrix metalloproteinase activity. BMB Rep. 2008, 41, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Emonard, H.; Bellon, G.; de Mettlen, M.; Hornebeck, W.; Courtoy, P.J. Regulation of matrix metalloproteinase (MMP) activity by the low-density lipoprotein receptor-related protein (LRP). A new function for an “old friend”. Biochimie 2005, 87, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Barcelona, P.F.; Jaldín-Fincati, J.R.; Sánchez, M.C.; Chiabrando, G.A. Activated α2-macroglobulin induces müller glial cell migration by regulating MT1-MMP activity through LRP1. J. Exp. Biol. 2013, 27, 3181–3197. [Google Scholar]
- Oh, J.; Takahashi, R.; Kondo, S.; Mizoguchi, A.; Adachi, E.; Sasahara, R.M. The membrane-anchored mmp inhibitor RECK is a key regulator of extracellular matrix integrity and angiogenesis. Cell 2001, 107, 789–800. [Google Scholar] [CrossRef]
- Miki, T.; Takegami, Y.; Okawa, K.; Muraguchi, T.; Noda, M.; Takahashi, C. The reversion-inducing cysteine-rich protein with Kazal motifs (RECK) interacts with membrane type 1 matrix metalloproteinase and CD13/aminopeptidase N and modulates their endocytic pathways. J. Bio. Chem. 2007, 282, 12341–12352. [Google Scholar] [CrossRef] [PubMed]
- Yeo, E.S.; Hwang, J.Y.; Park, J.E.; Choi, Y.J.; Huh, K.B.; Kim, W.Y. Tumor necrosis factor (TNF-α) and C-reactive protein (CRP) are positively associated with the risk of chronic kidney disease in patients with type 2 diabetes. Yonsei Med. J. 2010, 51, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Xie, J.; Chen, R.; Wang, C.; Xu, C.; Chen, C. Effect of titanium particles and TNF-α on the gene expression and activity of MMP-1, 2, 3 in human knee joint synovial cells. J. Bio. Eng. 2013, 30, 1022–1026. [Google Scholar]
- Lee, C.W.; Lin, C.C.; Lin, W.N.; Liang, K.C.; Luo, S.F.; Wu, C.B. TNF-α induces MMP-9 expression via activation of SCR/EGFR, PDGFR/PI3K/AKT cascade and promotion of NF-κB/P300 binding in human tracheal smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.P.; Tuan, T.L.; Wu, H.; Hughes, M.; Garner, W.L. TNF-α stimulates activation of pro-MMP2 in human skin through NF-κB mediated induction of MT1-MMP. J. Cell Sci. 2001, 114, 131–139. [Google Scholar] [PubMed]
- Wang, Y.; Tang, Z.; Xue, R.; Singh, G.K.; Shi, K.; Lv, Y. Combined effects of TNF-α, IL-1β, and HIF-1α on MMP-2 production in ACL fibroblasts under mechanical stretch: An in vitro study. J. Orthop. Res. 2011, 29, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Samanta, K.; Chakraborti, T.; Chowdhury, A.; Chakraborti, S. Role of TGF-β1 and TNF-α in IL-1β mediated activation of pro-MMP-9 in pulmonary artery smooth muscle cells: Involvement of an aprotinin sensitive protease. Arch. Biochem. Biophys. 2011, 513, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Gearing, A.J.; Beckett, P.; Christodoulou, M.; Churchill, M.; Clements, J.M.; Crimmin, M.; Davidson, A.H.; Drummond, A.H.; Galloway, W.A.; Gilbert, R. Matrix metalloproteinases and processing of pro-TNF-α. J. Leukocyte Biol. 1995, 57, 774–777. [Google Scholar] [PubMed]
- Murea, M.; Register, T.C.; Divers, J.; Bowden, D.W.; Carr, J.J.; Hightower, C.R. Relationships between serum MCP-1 and subclinical kidney disease: African American-diabetes heart study. BMC Nephrol. 2012, 13, 148. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Ishiguro, H.; Kobayashi, N.; Hasumi, H.; Watanabe, M.; Yao, M. Adipocyte-derived monocyte chemotactic protein-1 (MCP-1) promotes prostate cancer progression through the induction of MMP-2 activity. Prostate 2015, 75, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Q.; Li, W.; Li, S.Q.; Li, J.; Li, Y.W.; Kong, S.X. MCP-1 stimulates MMP-9 expression via ERK 1/2 and p38 MAPK signaling pathways in human aortic smooth muscle cells. Cell. Physiol. Biochem. 2014, 34, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Westermann, D.; Savvatis, K.; Lindner, D.; Zietsch, C.; Becher, P.M.; Hammer, E. Reduced degradation of the chemokine MCP-3 by matrix metalloproteinase-2 exacerbates myocardial inflammation in experimental viral cardiomyopathy. Circulation 2011, 124, 2082–2093. [Google Scholar] [CrossRef] [PubMed]
- Mcquibban, G.A.; Gong, J.H.; Tam, E.M.; Mcculloch, C.A.; Clark-Lewis, I.; Overall, C.M. Inflammation dampened by gelatinase a cleavage of monocyte chemoattractant protein-3. Science 2000, 289, 1202–1205. [Google Scholar] [CrossRef] [PubMed]
- Fernandezpatron, C.; Leung, D. Emergence of a metalloproteinase/phospholipase A2 axis of systemic inflammation. Metalloprotein. Med. 2015, 2, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.A.; Katz, R.; Shlipak, M.; Dubin, R.; Deboer, I.; Jenny, N. Kidney function decline in the elderly: Impact of lipoprotein-associated phospholipase A2. Am. J. Nephrol. 2011, 34, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, S.S.; Na, S.P.; Yu, C.Y.; Ji, Y.; Zhao, S.L. Characterization of lipoprotein-associated phospholipase A2 in serum in patients with stage 3–5 chronic kidney disease. Am. J. Med. Sci. 2016, 352, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Liu, S.; Zhao, Z.; Liu, Y.; Li, T. The effect of connective tissue growth factor on renal fibrosis and podocyte injury in hypertensive rats. Ren. Fail. 2014, 36, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Gao, W.; Dang, Y.; Liu, X.; Li, Y.; Peng, X. Both ERK/MAPK and TGF-β/Smad signaling pathways play a role in the kidney fibrosis of diabetic mice accelerated by blood glucose fluctuation. J. Diabetes Res. 2013, 463740. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Peng, K.; Qiu, C.; Skibba, M.; Huang, Y.; Xu, Z. Novel EGFR inhibitor attenuates Ang II-induced Kidney Fibrosis. J. Pharmacol. Exp. Ther. 2015, 356, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Kellenberger, T.; Marcussen, N.; Nyengaard, J.R.; Wogensen, L.; Jespersen, B. Expression of hypoxia-inducible factor-1α and hepatocyte growth factor in development of fibrosis in the transplanted kidney. Transpl. Int. 2014, 28, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Dong, Y.; Tian, X.; Tan, T.K.; Liu, Z.; Zhao, Y. Matrix metalloproteinases contribute to kidney fibrosis in chronic kidney diseases. J. Nephrol. 2013, 2, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Levi, E.; Fridman, R.; Miao, H.Q.; Ma, Y.S.; Yayon, A.; Vlodavsky, I. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc. Natl. Acad. Sci. USA 1996, 93, 7069–7074. [Google Scholar] [CrossRef] [PubMed]
- Iekushi, K.; Taniyama, Y.; Azuma, J.; Sanada, F.; Dosaka, N.; Okayama, K. Hepatocyte growth factor attenuates renal fibrosis through myofibroblasts anoikis induced by FAK/MMP activaton. Circulation 2009, 120, 1054–1055. [Google Scholar]
- Ronco, P.; Lelongt, B.; Piedagnel, R.; Chatziantoniou, C. Matrix metalloproteinases in kidney disease progression and repair: A case of flipping the coin. Semin. Nephrol. 2007, 27, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Chen, L.; Fan, J.M.; Li, Z. Role of MMP-9 and TIMP-1 in renal tubular epithelial-myofibroblast transdifferentiation. J. Sichuan Univ. 2008, 39, 34–38. [Google Scholar]
- Curello, S.; Ceconi, C.; Giuli, F.D.; Panzali, A.F.; Milanesi, B.; Calarco, M. Oxidative stress during reperfusion of human hearts: Potential sources of oxygen free radicals. Cardiovasc. Res. 1995, 29, 118–125. [Google Scholar] [CrossRef]
- Tobar, N.; Villar, V.; Santibanez, J.F. Ros-NFkappab mediates TGF-β1-induced expression of urokinase-type plasminogen activator, matrix metalloproteinase-9 and cell invasion. Mol. Cell. Biochem. 2010, 340, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.Z.; Xian, W.Z.; Xian, F.C.; Ke, W.; Yi, P.C.; Yue, Y.D. Puerarin attenuated early diabetic kidney injury through down-regulation of matrix metalloproteinase 9 in streptozotocin-induced diabetic rats. PLoS ONE 2014, 9, e85690. [Google Scholar]
- Pulidoolmo, H.; Garcíaprieto, C.F.; ÁlvarezLlamas, G.; Barderas, M.G.; Vivanco, F.; Aranguez, I. Role of matrix metalloproteinase (MMP)-9 in chronic kidney disease: A new biomarker of resistant albuminuria. Clin. Sci. 2016, 130, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Valentin, F.; Bueb, J.L.; Kieffer, A.P.; Tschirhart, B.E.; Atkinson, A.J. Oxidative stress activates MMP-2 in cultured human coronary smooth muscle cells. Fundam. Clin. Pharmacol. 2005, 19, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Godefroy, E.; Gallois, A.; Idoyaga, J.; Merad, M.; Tung, N.; Monu, N. Activation of toll-like receptor-2 by endogenous matrix metalloproteinase-2 modulates dendritic-cell-mediated inflammatory responses. Cell Rep. 2014, 9, 1856–1870. [Google Scholar] [CrossRef] [PubMed]
- Chandramu, C.; Sajani, S.; Lakka, P.B; Jasti, S.R. MMP-2 alters VEGF expression via αVβ3 integrin-mediated PI3K/AKT signaling in A549 lung cancer cells. Int. J. Cancer 2010, 127, 1081–1095. [Google Scholar]
- Higgins, D.F.; Biju, M.P.; Akai, Y.; Wutz, A.; Johnson, R.S.; Haase, V.H. Hypoxic induction of CTGF is directly mediated by HIF-1. Am. J. Physiol. Ren. Physiol. 2004, 287, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- O’Seaghdha, C.M. Lower urinary connective tissue growth factor levels and incident CKD stage 3 in the general population. Am. J. Kidney Dis. 2011, 57, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Qi, W.; Pollock, C.A. CTGF and chronic kidney fibrosis. Front. Biosci. 2009, 1, 132–141. [Google Scholar] [CrossRef]
- Elisabetta, M.; Gen, I.; Xiao, Q.L.; Kazuhisa, T.; Alban, G.; Steven, L.; Gonias, W.M.C. The hemopexin domain of matrix metalloproteinase-9 activates cell-signaling and promotes migration of schwann cells by binding to low density lipoprotein receptor-related protein. J. Neurol. 2008, 28, 11571–11582. [Google Scholar]
- D’Alessio, S.; Ferrari, G.; Cinnante, K.; Scheerer, W.; Galloway, A.C.; Roses, D.F. Tissue inhibitor of metalloproteinases-2 binding to membrane-type 1 matrix metalloproteinase induces MAPK activation and cell growth by a non-proteolytic mechanism. J. Biol. Chem. 2008, 283, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Seiki, M. Cytoplasmic tail of MT1-MMP regulates macrophage motility independently from its protease activity. Genes Cells 2009, 14, 617–626. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Limbu, M.H.; Wang, Z.; Liu, J.; Liu, L.; Zhang, X.; Chen, P.; Liu, B. MMP-2 and 9 in Chronic Kidney Disease. Int. J. Mol. Sci. 2017, 18, 776. https://doi.org/10.3390/ijms18040776
Cheng Z, Limbu MH, Wang Z, Liu J, Liu L, Zhang X, Chen P, Liu B. MMP-2 and 9 in Chronic Kidney Disease. International Journal of Molecular Sciences. 2017; 18(4):776. https://doi.org/10.3390/ijms18040776
Chicago/Turabian StyleCheng, Zhengyuan, Manoj Hang Limbu, Zhi Wang, Jing Liu, Lei Liu, Xiaoyi Zhang, Pingsheng Chen, and Bicheng Liu. 2017. "MMP-2 and 9 in Chronic Kidney Disease" International Journal of Molecular Sciences 18, no. 4: 776. https://doi.org/10.3390/ijms18040776
APA StyleCheng, Z., Limbu, M. H., Wang, Z., Liu, J., Liu, L., Zhang, X., Chen, P., & Liu, B. (2017). MMP-2 and 9 in Chronic Kidney Disease. International Journal of Molecular Sciences, 18(4), 776. https://doi.org/10.3390/ijms18040776