Large-Scale Membrane- and Lignin-Modified Adsorbent-Assisted Extraction and Preconcentration of Triazine Analogs and Aflatoxins
Abstract
:1. Introduction
2. Results and Discussion
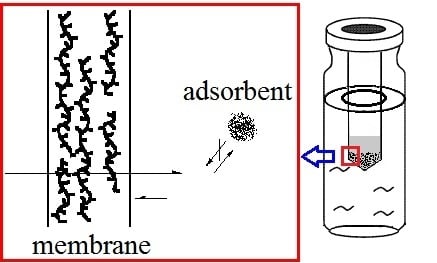
2.1. Effect of the Characteristics and Thickness of the Membrane on Permeation
2.2. Viscosity and Polarity Effects
2.3. Factors Affecting the Extraction Process and Adsorption Capacity Measurement
2.4. Adsorbent Amount and the Extraction Efficiency
2.5. Theoretical Interaction Simulation and FTIR Data
3. Experimental Procedures
3.1. Apparatus
3.2. Chemicals
3.3. Conditions for Solvent Permeation and Volume Loss Evaluations
3.4. Conditions for Measuring the Percentage of Extraction of Analyte
3.5. Conditions for Measuring the Percentage of Adsorption and the Adsorption Capacity
3.6. Theoretical Computational Calculation with Spartan’14 Software
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rippen, G. Handbuch Limueltchemikalien, 3rd ed.; 16 Erg Lfg.; Ecomed: Landsberg/Lech, Germany, 1992. [Google Scholar]
- Environmental Protection Agency. Cyanazine; notice of final determination to terminate special review of cyanazine; notice of voluntary cancellation and cancellation order of cyanazine product registrations. Federal Register 1996, 61, 39024–39029. [Google Scholar]
- Buser, H.R. Atrazine and other s-triazine herbicides in lakes and in rain in Switzerland. Environ. Sci. Technol. 1990, 24, 1049–1058. [Google Scholar] [CrossRef]
- Bann, B.; Miller, S.A. Melamine and derivatives of melamine. Chem. Rev. 1958, 58, 131–172. [Google Scholar] [CrossRef]
- Lim, L.O.; Scherer, S.J.; Shuler, K.D.; Toth, J.P. Disposition of cyromazine in plants under environmental conditions. J. Agric. Food Chem. 1990, 38, 860–864. [Google Scholar] [CrossRef]
- Dobson, R.L.; Motlagh, S.; Quijano, M.; Cambron, R.T.; Baker, T.R.; Pullen, A.M.; Regg, B.T.; Bigalow-Kern, A.S.; Vennard, T.; Fix, A. Identification and characterization of toxicity of contaminants in pet food leading to an outbreak of renal toxicity in cats and dogs. Toxicol. Sci. 2008, 106, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C. Veterinary Toxicology, 2nd ed.; Academic Press: Waltham, MA, USA, 2012; Chapter 96; pp. 1257–1280. [Google Scholar]
- Huang, P.; Yang, J.; Song, Q. Atrazine affects phosphoprotein and protein expression in MCF-10A human breast epithelial cells. Int. J. Mol. Sci. 2014, 15, 17806–17826. [Google Scholar] [CrossRef] [PubMed]
- Terman, G.; DeMent, J.; Hunt, C.; Cope, J., Jr.; Ensminger, L. Fertilizer nitrogen sources, crop response to urea and urea pyrolysis products. J. Agric. Food Chem. 1964, 12, 151–154. [Google Scholar] [CrossRef]
- González-Rodríguez, R.; Rial-Otero, R.; Cancho-Grande, B.; Gonzalez-Barreiro, C.; Simal-Gándara, J. A review on the fate of pesticides during the processes within the food-production chain. Crit. Rev. Food Sci. Nutr. 2011, 51, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Corcia, A.D.; Marchetti, M. Method development for monitoring pesticides in environmental waters: Liquid-solid extraction followed by liquid chromatography. Environ. Sci. Technol. 1992, 26, 66–74. [Google Scholar] [CrossRef]
- Di Corcia, A.; Marchetti, M. Multiresidue method for pesticides in drinking water using a graphitized carbon black cartridge extraction and liquid chromatographic analysis. Anal. Chem. 1991, 63, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.T.; Cohen, Y.; Kaplan, I.R. Intermedia Pollution Transport: Modelling and Field Measurements; Plenum: New York, NY, USA, 1989. [Google Scholar]
- López-Blanco, C.; Gómez-Álvarez, S.; Rey-Garrote, M.; Cancho-Grande, B.; Simal-Gándara, J. Determination of pesticides by solid phase extraction followed by gas chromatography with nitrogen–phosphorous detection in natural water and comparison with solvent drop microextraction. Anal. Bioanal. Chem. 2006, 384, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, M.M.; Mueller, S.R.; Singer, H.P.; Imboden, D.M.; Schwarzenbach, R.P. Input and dynamic behavior of the organic pollutants tetrachloroethene, atrazine, and NTA in a lake: A study combining mathematical modeling and field measurements. Environ. Sci. Technol. 1994, 28, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Kolpin, D.W.; Kalkhoff, S.J. Atrazine degradation in a small stream in Iowa. Environ. Sci. Technol. 1993, 27, 134–139. [Google Scholar] [CrossRef]
- Hauser, B.; Schellin, M.; Popp, P. Membrane-assisted solvent extraction of triazines, organochlorine, and organophosphorus compounds in complex samples combined with large-volume injection-gas chromatography/mass spectrometric detection. Anal. Chem. 2004, 76, 6029–6038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wong, J.W.; Yang, P.; Tech, K.; DiBenedetto, A.L.; Lee, N.S.; Hayward, D.G.; Makovi, C.M.; Krynitsky, A.J.; Banerjee, K. Multiresidue pesticide analysis of agricultural commodities using acetonitrile salt-out extraction, dispersive solid-phase sample clean-up, and high-performance liquid chromatography–tandem mass spectrometry. J. Agric. Food Chem. 2011, 59, 7636–7646. [Google Scholar] [CrossRef] [PubMed]
- Koeber, R.; Fleischer, C.; Lanza, F.; Boos, K.-S.; Sellergren, B.; Barceló, D. Evaluation of a multidimensional solid-phase extraction platform for highly selective on-line cleanup and high-throughput LC-MS analysis of triazines in river water samples using molecularly imprinted polymers. Anal. Chem. 2001, 73, 2437–2444. [Google Scholar] [CrossRef] [PubMed]
- Matsui, J.; Goji, S.; Murashima, T.; Miyoshi, D.; Komai, S.; Shigeyasu, A.; Kushida, T.; Miyazawa, T.; Yamada, T.; Tamaki, K. Molecular imprinting under molecular crowding conditions: An aid to the synthesis of a high-capacity polymeric sorbent for triazine herbicides. Anal. Chem. 2007, 79, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Lanza, F.; Tolokan, A.; Horvath, V.; Sellergren, B.; Horvai, G.; Barceló, D. Selective trace enrichment of chlorotriazine pesticides from natural waters and sediment samples using terbuthylazine molecularly imprinted polymers. Anal. Chem. 2000, 72, 3934–3941. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bai, L.-Y.; Liu, K.-F.; Liu, R.-Q.; Zhang, Y.-P. Atrazine molecular imprinted polymers: Comparative analysis by far-infrared and ultraviolet induced polymerization. Int. J. Mol. Sci. 2014, 15, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Pensabene, J.W.; Fiddler, W.; Donoghue, D.J. Supercritical fluid extraction of atrazine and other triazine herbicides from fortified and incurred eggs. J. Agric. Food Chem. 2000, 48, 1668–1672. [Google Scholar] [CrossRef] [PubMed]
- Carabias-Martinez, R.; Rodriguez-Gonzalo, E.; Dominguez-Alvarez, J.; Hernández-Méndez, J. Cloud point extraction as a preconcentration step prior to capillary electrophoresis. Anal. Chem. 1999, 71, 2468–2474. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Xiao, X.; Cheng, H.; Zhou, Z.; Sheng, G.D. Influence of environmental factors on pesticide adsorption by black carbon: pH and model dissolved organic matter. Environ. Sci. Technol. 2009, 43, 4973–4978. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chun, Y.; Sheng, G.; Huang, M. pH-dependence of pesticide adsorption by wheat-residue-derived black carbon. Langmuir. 2004, 20, 6736–6741. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, I.; Albanis, T. Adsorption-desorption studies of selected herbicides in soil-fly ash mixtures. J. Agric. Food Chem. 2000, 48, 4780–4790. [Google Scholar] [CrossRef] [PubMed]
- Arias-Estévez, M.; Soto-González, B.; López-Periago, E.; Cancho-Grande, B.; Simal-Gándara, J. Atrazine sorption dynamics in acid-surface soils. Bull. Environ. Contam. Toxicol. 2005, 75, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.; Sultana, B.; Anwar, F.; Khan, M.Z.; Ashrafuzzaman, M. Occurrence of aflatoxins in selected processed foods from Pakistan. Int. J. Mol. Sci. 2012, 13, 8324–8337. [Google Scholar] [CrossRef] [PubMed]
- Nilüfer, D.; Boyacıoǧlu, D. Comparative study of three different methods for the determination of aflatoxins in tahini. J. Agric. Food Chem. 2002, 50, 3375–3379. [Google Scholar] [CrossRef] [PubMed]
- Shephard, G.S. Aflatoxin analysis at the beginning of the twenty-first century. Anal. Bioanal. Chem. 2009, 395, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Khayoon, W.S.; Saad, B.; Yan, C.B.; Hashim, N.H.; Ali, A.S.M.; Salleh, M.I.; Salleh, B. Determination of aflatoxins in animal feeds by HPLC with multifunctional column clean-up. Food Chem. 2010, 118, 882–886. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Jinap, S.; Arzandeh, S.; Mirhosseini, H. Optimization of HPLC conditions for quantitative analysis of aflatoxins in contaminated peanut. Food Control 2011, 22, 381–388. [Google Scholar] [CrossRef]
- Wei, R.; Qiu, F.; Kong, W.; Wei, J.; Yang, M.; Luo, Z.; Qin, J.; Ma, X. Co-occurrence of aflatoxin B 1, B 2, G 1, G 2 and ochrotoxin A in Glycyrrhiza uralensis analyzed by HPLC-MS/MS. Food Control 2013, 32, 216–221. [Google Scholar] [CrossRef]
- McCullum, C.; Tchounwou, P.; Ding, L.-S.; Liao, X.; Liu, Y.-M. Extraction of aflatoxins from liquid foodstuff samples with polydopamine-coated superparamagnetic nanoparticles for HPLC-MS/MS analysis. J. Agric. Food Chem. 2014, 62, 4261–4267. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.W.; Sun, D.L.; Ruan, C.Q.; Zhang, H.; Liu, C.L. Rapid analysis of aflatoxins B1, B2, and ochratoxin A in rice samples using dispersive liquid–liquid microextraction combined with HPLC. J. Sep. Sci. 2014, 37, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, A. Chemical structure of lignin related mainly to degradation products. In Recent Advances in Lignin Biodegradation Research; Higuchi, T., Chang, H.M., Kirk, T.K., Eds.; UNI Publisher: Tokyo, Japan, 1983; pp. 12–33. [Google Scholar]
- Fang, Z.; Smith, R.L., Jr. Production of Biofuels and Chemicals from Lignin; Springer Science + Business Media Singapore: Singapore, 2016; Chapter 1; pp. 3–33. [Google Scholar]
- Valencia, Z.; Chavez, E. Lignin as a purified dietary fiber supplement for piglets. Nutr. Res. 1997, 17, 1517–1527. [Google Scholar] [CrossRef]
- Kay, R. Dietary fiber. J. Lipid Res. 1982, 23, 221–242. [Google Scholar] [PubMed]
- He, Z.-W.; He, L.-H.; Yang, J.; Lü, Q.-F. Removal and recovery of Au (III) from aqueous solution using a low-cost lignin-based biosorbent. Ind. Eng. Chem. Res. 2013, 52, 4103–4108. [Google Scholar] [CrossRef]
- Hu, S.W.; Chen, S. A Multipurpose Lignin-based Adsorbent for Metallic Ions, Nanoparticles and Various Organophosphate Pesticides in Hexane. J. Chin. Chem. Soc. 2015, 62, 875–888. [Google Scholar] [CrossRef]
- Alcudia-León, M.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Stir membrane extraction: A useful approach for liquid sample pretreatment. Anal. Chem. 2009, 81, 8957–8961. [Google Scholar] [CrossRef] [PubMed]
- Bedendo, G.C.; Jardim, I.C.S.F.; Carasek, E. A simple hollow fiber renewal liquid membrane extraction method for analysis of sulfonamides in honey samples with determination by liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 6449–6454. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Y.; Jiang, H.; Zhao, G. A novel method for determination and quantification of 4-methyloctanoic and 4-methylnonanoic acids in mutton by hollow fiber supported liquid membrane extraction coupled with gas chromatography. Meat Sci. 2012, 92, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Cukrowska, E.; Chimuka, L.; Nsengimana, H.; Kwaramba, V. Application of supported liquid membrane probe for extraction and preconcentration of organotin compounds from environmental water samples. Anal. Chim. Acta 2004, 523, 141–147. [Google Scholar] [CrossRef]
- Grzenia, D.L.; Dong, R.W.; Jasuja, H.; Kipper, M.J.; Qian, X.; Wickramasinghe, S.R. Conditioning biomass hydrolysates by membrane extraction. J. Membr. Sci. 2012, 415, 75–84. [Google Scholar] [CrossRef]
- Grzenia, D.L.; Schell, D.J.; Wickramasinghe, S.R. Membrane extraction for removal of acetic acid from biomass hydrolysates. J. Membr. Sci. 2008, 322, 189–195. [Google Scholar] [CrossRef]
- Kumrić, K.R.; Vladisavljević, G.T.; Trtić-Petrović, T.M. Membrane-assisted liquid-phase extraction of Lu (III) in a U-shaped contactor with a single hollow fiber membrane. Ind. Eng. Chem. Res. 2012, 51, 14199–14208. [Google Scholar] [CrossRef]
- Pantůčková, P.; Kubáň, P.; Boček, P. Sensitivity enhancement in direct coupling of supported liquid membrane extractions to capillary electrophoresis by means of transient isotachophoresis and large electrokinetic injections. J. Chromatogr. A 2015, 1389, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Poliwoda, A.; Krzyżak, M.; Wieczorek, P.P. Supported liquid membrane extraction with single hollow fiber for the analysis of fluoroquinolones from environmental surface water samples. J. Chromatogr. A 2010, 1217, 3590–3597. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kou, D.; Mitra, S. Continuous, on-line monitoring of haloacetic acids via membrane extraction. J. Chromatogr. A 2005, 1089, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cai, C.; Lin, Y.; Bian, Y.; Guo, H.; Chen, X. Enantioselective separation of ketoconazole enantiomers by membrane extraction. Sep. Purif. Technol. 2011, 79, 63–71. [Google Scholar] [CrossRef]
- Basualto, C.; Marchese, J.; Valenzuela, F.; Acosta, A. Extraction of molybdenum by a supported liquid membrane method. Talanta 2003, 59, 999–1007. [Google Scholar] [CrossRef]
- Hu, S.-W.; Chen, S. Adsorption of triazine derivatives with humic fraction-immobilized silica gel in hexane: A mechanistic consideration. J. Agric. Food Chem. 2013, 61, 8524–8532. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-S.; Chen, S. Adsorption of Pesticidal Compounds Bearing a Single Carboxyl Functional Group and Biogenic Amines by Humic Fraction-Immobilized Silica Gel. J. Agric. Food Chem. 2013, 61, 3600–3610. [Google Scholar] [CrossRef] [PubMed]
- Leloup, M.; Pallier, V.; Nicolau, R.; Feuillade-Cathalifaud, G. Assessing Transformations of Algal Organic Matter in the Long-Term: Impacts of Humification-Like Processes. Int. J. Mol. Sci. 2015, 16, 18096–18110. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Bloxham, S.; Eicher-Lorka, O.; Jakubënas, R.; Niaura, G. Surface-enhanced Raman spectroscopy of ethanethiol adsorbed at copper electrode. Chemija 2002, 13, 185–189. [Google Scholar]
- Armstrong, D.W.; Tang, Y.; Chen, S.; Zhou, Y.; Bagwill, C.; Chen, J.-R. Macrocyclic antibiotics as a new class of chiral selectors for liquid chromatography. Anal. Chem. 1994, 66, 1473–1484. [Google Scholar] [CrossRef]
- Hsiao, Y.-L.; Chen, S. LC separation of enantiomers on silica-bonded thiostrepton derivatives. Chromatographia 2009, 70, 1031–1038. [Google Scholar] [CrossRef]








| Solvent | Vapor Pressure a (mmHg) | Dielectric Constant | Water Solubility | Boiling Point (°C) | Viscosity (mPa·s) |
|---|---|---|---|---|---|
| Water | 23.7 | 80.10 | - | 100 | 0.89 |
| Hexane | 151.2 | 1.88 | 9.5 mg/L | 68.7 | 0.29 |
| Cyclohexane | 97.6 | 2.02 | 55 mg/L | 80.7 | 0.90 |
| Ethanol | 58.8 | 24.30 | 1 kg/L | 78.2 | 1.07 |
| Diethyl ether | 532.7 | 4.33 | 60.4 g/L | 34.6 | 0.22 |
| Acetonitrile | 91.2 | 37.5 | 1 kg/L | 81.6 | 0.34 |
| Dichloromethane | 352.5 | 9.1 | 13 g/L | 39 | 0.43 |
| No. | Compound a | Analyte Structure | Percent b Extraction (%) | Percent c Adsorption (%) | Adsorption d Capacity (mg) | Solubility e (H2O/HEX) |
|---|---|---|---|---|---|---|
| 1 | Prometon |  | 42.23 (1) 41.52 (3) 47.47/77.13 * (2) | ~100 | - | 620/12,000 - |
| 2 | Anilazine |  | 62.13 (1) 55.06 (3) 84.88/~100 * (2) | ~100 | 0.419 | 8/1700 8/- |
| 3 | Ametryne |  | 87.70 (1) 90.10 (3) ~100 (2) | ~100 | 0.429 | 200/14,000 209/- |
| 4 | Tebuthiuron * |  | 19.66 (1) 27.72 (3) 57.03/86.47 * (2) | ~100 | - | - 2500/6100 |
| 5 | Atraton |  | 20.97 (1) 28.56 (3) 41.93/67.12 * (2) | ~100 | - | 1800/- - |
| 6 | Prometryne |  | 88.60 (1) 98.35 (3) ~100 (2) | ~100 | 0.417 | -/5500 33/6300 |
| 7 | Terbutryne |  | 49.36 (1) 86.47 (3) ~100 (2) | ~100 | 0.424 | 25/9000 - |
| 8 | Metribuzin |  | 49.14 (1) 51.43 (3) 63.37/89.74 * (2) | ~100 | - | 1050/1000 - |
| 9 | Terbumeton |  | ~100 (1) ~100 (3) ~100 (2) | ~100 | 0.432 | 130/220,000 - |
| 10 | Atrazine |  | 29.59 (1) 7.70 (3) 28.83/55.56 * (2) | ~100 | - | 28/- 33/110 |
| 11 | Methoprotryne |  | 49.09 (1) 59.56 (3) 63.56/91.48 * (2) | ~100 | - | 320/5000 - |
| 12 | Dipropetryn |  | ~100 (1) ~100 (3) ~100 (2) | ~100 | 0.426 | 16/9000 - |
| 13 | Simetryn |  | 38.04 (1) 42.43 (3) 54.10/79.68 * (2) | ~100 | - | 400/4000 - |
| 14 | Procyazine |  | 2.33 (1) 1.54 (3) 1.05/- (2) | ~100 | - | -/50 248/- |
| No. | Compound | Analyte Structure | Percent a Extraction (%) | Percent b Adsorption (%) | Adsorption c Capacity (mg) | Solubility d H2O/Solvent |
|---|---|---|---|---|---|---|
| 1 | Aflatoxin G1 |  | 41.76/68.55 (1) 50.14/83.13 (2) | ~100 | 0.290 | (10–20) × 10−3/5 |
| 2 | Aflatoxin G2 |  | 40.76/71.94 (1) 51.13/82.37 (2) | ~100 | 0.293 | (10–20) × 10−3/5 |
| 3 | Aflatoxin B1 |  | 39.56/69.72 (1) 49.82/81.64 (2) | ~100 | 0.297 | (10–20) × 10−3/10 |
| 4 | Aflatoxin B2 |  | 37.56/66.97 (1) 47.03/80.17 (2) | ~100 | 0.295 | (10–20) × 10−3/5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.-W.; Chen, S. Large-Scale Membrane- and Lignin-Modified Adsorbent-Assisted Extraction and Preconcentration of Triazine Analogs and Aflatoxins. Int. J. Mol. Sci. 2017, 18, 801. https://doi.org/10.3390/ijms18040801
Hu S-W, Chen S. Large-Scale Membrane- and Lignin-Modified Adsorbent-Assisted Extraction and Preconcentration of Triazine Analogs and Aflatoxins. International Journal of Molecular Sciences. 2017; 18(4):801. https://doi.org/10.3390/ijms18040801
Chicago/Turabian StyleHu, Shun-Wei, and Shushi Chen. 2017. "Large-Scale Membrane- and Lignin-Modified Adsorbent-Assisted Extraction and Preconcentration of Triazine Analogs and Aflatoxins" International Journal of Molecular Sciences 18, no. 4: 801. https://doi.org/10.3390/ijms18040801
APA StyleHu, S. -W., & Chen, S. (2017). Large-Scale Membrane- and Lignin-Modified Adsorbent-Assisted Extraction and Preconcentration of Triazine Analogs and Aflatoxins. International Journal of Molecular Sciences, 18(4), 801. https://doi.org/10.3390/ijms18040801