Preventive Effects of Heat-Killed Enterococcus faecalis Strain EC-12 on Mouse Intestinal Tumor Development
Abstract
:1. Introduction
2. Results
2.1. Suppression of Intestinal Polyp Formation in Min Mice by Heat-Killed EC-12
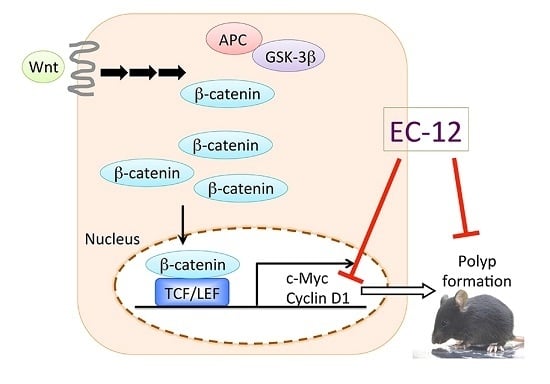
2.2. Weak Suppression of Gene Expression Regulated by β-Catenin Signaling in the Intestinal Polyps of Min Mice by Heat-Killed EC-12
2.3. Suppression of TCF/LEF Promoter Transcriptional Activity by Heat-Killed EC-12
2.4. No Obvious Changes Were Observed in the Enterobacterium with and without Heat-Killed EC-12 Treatment
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Animals
4.4. Animal Experimental Protocol
4.5. Bacterial DNA Extraction from Feces
4.6. Luciferase Assays for TCF/LEF Promoter Transcriptional Activity in Stable Transfectants
4.7. Quantitative Real-Time PCR Analysis for Tissue Sample Evaluation
4.8. Quantitative Real-Time PCR Analysis for Enterobacterium Evaluation
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Conflicts of Interest
Abbreviations
| AOM | Azoxymethane |
| COX | Cyclooxygenase |
| CRC | Colorectal cancer |
| DSS | Dextran sodium sulfate |
| EC-12 | Enterococcus faecalis strain EC-12 |
| FBS | Fetal bovine serum |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| LAB | Lactic acid bacteria |
| PCR | Polymerase chain reaction |
| PPAR | Peroxisome proliferator-activated receptor |
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; World Cancer Research Fund/American Institute for Cancer Research: Washington DC, USA, 2007. [Google Scholar]
- Cross, M.L.; Stevenson, L.M.; Gill, H.S. Anti-allergy properties of fermented foods: An important immunoregulatory mechanism of lactic acid bacteria? Int. Immunopharmacol. 2001, 1, 891–901. [Google Scholar] [CrossRef]
- Inoue, R.; Nishio, A.; Fukushima, Y.; Ushida, K. Oral treatment with probiotic Lactobacillus johnsonii NCC533 (La1) for a specific part of the weaning period prevents the development of atopic dermatitis induced after maturation in model mice, NC/Nga. Br. J. Dermatol. 2007, 156, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Reid, G.; Bruce, A.W.; McGroarty, J.A.; Cheng, K.J.; Costerton, J.W. Is there a role for lactobacilli in prevention of urogenital and intestinal infections? Clin. Microbial. Rev. 1990, 3, 335–344. [Google Scholar] [CrossRef]
- Asahara, T.; Shimizu, K.; Nomoto, K.; Hamabata, T.; Ozawa, A.; Takeda, Y. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect. Immun. 2004, 72, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Murosaki, S.; Muroyama, K.; Yamamoto, Y.; Yoshikai, Y. Antitumor effect of heat-killed Lactobacillus plantarum L-137 through restoration of impaired interleukin-12 production in tumor-bearing mice. Cancer Immunol. Immunother. CII 2000, 49, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Sawada, J.; Morita, H.; Tanaka, A.; Salminen, S.; He, F.; Matsuda, H. Ingestion of heat-treated Lactobacillus rhamnosus GG prevents development of atopic dermatitis in NC/Nga mice. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2007, 37, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Tobita, K.; Yanaka, H.; Otani, H. Heat-treated Lactobacillus crispatus KT strains reduce allergic symptoms in mice. J. Agric. Food Chem. 2009, 57, 5586–5590. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Kawase, M.; He, F.; Kubota, A.; Yoda, K.; Miyazawa, K.; Hiramatsu, M. Heat-killed Lactobacillus gasseri TMC0356 protects mice against influenza virus infection by stimulating gut and respiratory immune responses. FEMS Immunol. Med. Microbiol. 2012, 64, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Vacheron, F.; Guenounou, M.; Nauciel, C. Induction of interleukin 1 secretion by adjuvant-active peptidoglycans. Infect. Immun. 1983, 42, 1049–1054. [Google Scholar] [PubMed]
- Humphrey, B.D.; Huang, N.; Klasing, K.C. Rice expressing lactoferrin and lysozyme has antibiotic-like properties when fed to chicks. J. Nutr. 2002, 132, 1214–1418. [Google Scholar] [PubMed]
- Nishibayashi, R.; Inoue, R.; Harada, Y.; Watanabe, T.; Makioka, Y.; Ushida, K. RNA of Enterococcus faecalis strain EC-12 is a major component inducing interleukin-12 production from human monocytic cells. PLoS ONE 2015, 10, e0129806. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Tsukahara, T.; Matsukawa, N.; Watanabe, T.; Bukawa, W.; Nakayama, K.; Ushida, K. Rapid induction of an immune response in rat Peyer’s patch after oral administration of Enterococcus faecalis strain EC-12. Biosci. Biotechnol. Biochem. 2013, 77, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Inoue, R.; Nakanishi, N.; Nakayama, K.; Matsubara, N.; Ushida, K. Evaluation of the low dose level of a heat-killed and dried cell preparation of Enterococcus faecalis to prevent porcine edema disease using experimental infection model with enterotoxcemic Escherichia coli in weaning pigs. J. Vet. Med. Sci. 2007, 69, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, N.; Reddy, B.S. Inhibitory effect of Bifidobacterium longum cultures on the azoxymethane-induced aberrant crypt foci formation and fecal bacterial β-glucuronidase. Proc. Soc. Exp. Biol. Med. 1994, 207, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Rivenson, A.; Tomita, M.; Shimamura, S.; Ishibashi, N.; Reddy, B.S. Bifidobacterium longum, a lactic acid-producing intestinal bacterium inhibits colon cancer and modulates the intermediate biomarkers of colon carcinogenesis. Carcinogenesis 1997, 18, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y.; Suzuki, M.; Miyazaki, H.; Tsuboyama-Kasaoka, N.; Wu, J.; Ishimi, Y.; Ezaki, O. Ovariectomy in mice decreases lipid metabolism-related gene expression in adipose tissue and skeletal muscle with increased body fat. J. Nutr. Sci. Vitaminol. 2005, 51, 110–117. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, C.A.; Barak, Y.; Matise, I.; Cormier, R.T. Intestinal-specific PPARγ deficiency enhances tumorigenesis in ApcMin/+ mice. Int. J. Cancer 2006, 119, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/β-catenin signaling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Niho, N.; Mutoh, M.; Takahashi, M.; Tsutsumi, K.; Sugimura, T.; Wakabayashi, K. Concurrent suppression of hyperlipidemia and intestinal polyp formation by NO-1886, increasing lipoprotein lipase activity in Min mice. Proc. Natl. Acad. Sci. USA 2005, 102, 2970–2974. [Google Scholar] [CrossRef] [PubMed]
- Niho, N.; Takahashi, M.; Shoji, Y.; Takeuchi, Y.; Matsubara, S.; Sugimura, T.; Wakabayashi, K. Dose-dependent suppression of hyperlipidemia and intestinal polyp formation in Min mice by pioglitazone, a PPAR γ ligand. Cancer Sci. 2003, 94, 960–964. [Google Scholar] [CrossRef]
- Niho, N.; Mutoh, M.; Komiya, M.; Ohta, T.; Sugimura, T.; Wakabayashi, K. Improvement of hyperlipidemia by indomethacin in Min mice. Int. J. Cancer 2007, 121, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Nakatsugi, S.; Fukutake, M.; Takahashi, M.; Fukuda, K.; Isoi, T.; Taniguchi, Y.; Sugimura, T.; Wakabayashi, K. Suppression of intestinal polyp development by nimesulide, a selective cyclooxygenase-2 inhibitor, in Min mice. Jpn. J. Cancer Res. Gann 1997, 88, 1117–1120. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Fujii, G.; Takahashi, M.; Nakanishi, R.; Komiya, M.; Shimura, M.; Noma, N.; Onuma, W.; Terasaki, M.; Yano, T.; et al. Sesamol suppresses cyclooxygenase-2 transcriptional activity in colon cancer cells and modifies intestinal polyp development in ApcMin/+ mice. J. Clin. Biochem. Nutr. 2014, 54, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Komiya, M.; Fujii, G.; Miyamoto, S.; Takahashi, M.; Ishigamori, R.; Onuma, W.; Ishino, K.; Totsuka, Y.; Fujimoto, K.; Mutoh, M. Suppressive effects of the NADPH oxidase inhibitor apocynin on intestinal tumorigenesis in obese KK-Ay and Apc mutant Min mice. Cancer Sci. 2015, 106, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Yoshida, Y.; Tsushima, T.; Watanabe, T.; Matsubara, N.; Inoue, R.; Ushida, K. Evaluation of the heat-killed and dried cell preparation of Enterococcus faecalis against villous atrophy in early-weaned mice and pigs. Anim. Sci. J. Nihon Chikusan Gakkaiho 2011, 82, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Songjinda, P.; Nakayama, J.; Tateyama, A.; Tanaka, S.; Tsubouchi, M.; Kiyohara, C.; Shirakawa, T.; Sonomoto, K. Differences in developing intestinal microbiota between allergic and non-allergic infants: A pilot study in Japan. Biosci. Biotechnol. Biochem. 2007, 71, 2338–2342. [Google Scholar] [CrossRef]
- Byun, R.; Nadkarni, M.A.; Chhour, K.L.; Martin, F.E.; Jacques, N.A.; Hunter, N. Quantitative analysis of diverse Lactobacillus species present in advanced dental caries. J. Clin. Microbial. 2004, 42, 3128–3136. [Google Scholar] [CrossRef] [PubMed]



| Dose (ppm) | Gender (Number of Mice) | Small Intestine | Colon | Total | ||
|---|---|---|---|---|---|---|
| Proximal | Middle | Distal | ||||
| 0 | Male (8) | 6.3 ± 4.3 | 9.3 ± 3.5 | 29.3 ± 7.6 | 1.8 ± 2.0 | 46.5 ± 11.6 |
| 50 | Male (10) | 6.7 ± 3.1 | 11.8 ± 6.4 | 25.7 ± 11.7 | 0.3 ± 0.7 | 44.5 ± 18.1 |
| 0 | Female (9) | 8.4 ± 5.2 | 12.2 ± 6.7 | 28.6 ± 15.5 | 0.1 ± 0.3 | 48.2 ± 19.7 |
| 50 | Female (8) | 3.8 ± 2.0 * | 10.0 ± 3.2 | 18.1 ± 11.3 | 0.8 ± 0.7 | 33.3 ± 13.9 |
| Dose (ppm) | Gender (Number of Mice) | Small Intestine | Colon | Total | ||
|---|---|---|---|---|---|---|
| Proximal | Middle | Distal | ||||
| 0 | Male (9) | 5.3 ± 2.1 | 9.3 ± 3.2 | 23.1 ± 2.9 | 1.4 ± 2.0 | 39.2 ± 4.2 |
| 100 | Male (9) | 2.3 ± 0.9 | 6.9 ± 2.9 * | 23.7 ± 8.3 | 0.8 ± 1.6 | 33.7 ± 11.7 |
| 0 | Female (8) | 5.3 ± 2.6 | 9.4 ± 2.8 | 25.9 ± 11.1 | 1.8 ± 3.5 | 42.3 ± 10.3 |
| 100 | Female (8) | 3.1 ± 2.0 | 8.5 ± 2.8 | 17.8 ± 9.3 | 0.4 ± 0.5 | 29.8 ± 13.0 |
| Dose (ppm) | Gender | Diameter (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <0.5 | 0.5 to <1.0 | 1.0 to <1.5 | 1.5 to <2.0 | 2.0 to <2.5 | 2.5 to <3.0 | 3.0 to <3.5 | 3.5 to <4.0 | 4.0 to <4.5 | ≥4.5 | ||
| 0 | Male | 13.4 ± 6.5 | 19.6 ± 7.1 | 7.0 ± 3.0 | 3.6 ± 2.9 | 2.0 ± 2.1 | 0.3 ± 0.5 | 0.3 ± 0.5 | 0.1 ± 0.4 | 0.3 ± 0.5 | 0.0 ± 0.0 |
| 50 | Male | 12.2 ± 6.1 | 21.2 ± 10.1 | 7.0 ± 6.1 | 3.1 ± 2.4 | 0.5 ± 0.7 | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.03 ± 0.2 |
| 0 | Female | 15.8 ± 7.2 | 23.7 ± 12.4 | 6.1 ± 4.7 | 1.6 ± 0.9 | 0.9 ± 0.8 | 0.1 ± 0.3 | 0.1 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 50 | Female | 8.3 ± 4.3 * | 14.9 ± 9.1 | 5.5 ± 2.1 | 3.3 ± 3.1 | 0.9 ± 0.6 | 0.1 ± 0.4 | 0.3 ± 0.5 | 0.0 ± 0.0 | 0.1 ± 0.4 | 0.0 ± 0.0 |
| Dose (ppm) | Gender | Diameter (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <0.5 | 0.5 to <1.0 | 1.0 to <1.5 | 1.5 to <2.0 | 2.0 to <2.5 | 2.5 to <3.0 | 3.0 to <3.5 | 3.5 to <4.0 | 4.0 to <4.5 | ≥4.5 | ||
| 0 | Male | 7.2 ± 4.4 | 16.6 ± 2.8 | 8.1 ± 3.6 | 4.3 ± 1.8 | 2.1 ± 0.8 | 0.8 ± 0.8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.3 |
| 100 | Male | 3.9 ± 4.2 | 12.9 ± 4.5 | 10.4 ± 4.9 | 4.6 ± 2.0 | 1.4 ± 1.0 | 0.1 ± 0.3 | 0.2 ± 0.4 | 0.1 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 0 | Female | 9.9 ± 4.3 | 20.9 ± 7.2 | 6.1 ± 3.0 | 2.5 ± 1.1 | 1.8 ± 1.4 | 0.6 ± 0.7 | 0.3 ± 0.5 | 0.0 ± 0.0 | 0.3 ± 0.5 | 0.0 ± 0.0 |
| 100 | Female | 3.9 ± 3.6 * | 12.5 ± 7.6 * | 7.9 ± 5.1 | 3.9 ± 2.0 | 1.1 ± 1.0 | 0.4 ± 0.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.1 ± 0.4 | 0.0 ± 0.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyamoto, S.; Komiya, M.; Fujii, G.; Hamoya, T.; Nakanishi, R.; Fujimoto, K.; Tamura, S.; Kurokawa, Y.; Takahashi, M.; Ijichi, T.; et al. Preventive Effects of Heat-Killed Enterococcus faecalis Strain EC-12 on Mouse Intestinal Tumor Development. Int. J. Mol. Sci. 2017, 18, 826. https://doi.org/10.3390/ijms18040826
Miyamoto S, Komiya M, Fujii G, Hamoya T, Nakanishi R, Fujimoto K, Tamura S, Kurokawa Y, Takahashi M, Ijichi T, et al. Preventive Effects of Heat-Killed Enterococcus faecalis Strain EC-12 on Mouse Intestinal Tumor Development. International Journal of Molecular Sciences. 2017; 18(4):826. https://doi.org/10.3390/ijms18040826
Chicago/Turabian StyleMiyamoto, Shingo, Masami Komiya, Gen Fujii, Takahiro Hamoya, Ruri Nakanishi, Kyoko Fujimoto, Shuya Tamura, Yurie Kurokawa, Maiko Takahashi, Tetsuo Ijichi, and et al. 2017. "Preventive Effects of Heat-Killed Enterococcus faecalis Strain EC-12 on Mouse Intestinal Tumor Development" International Journal of Molecular Sciences 18, no. 4: 826. https://doi.org/10.3390/ijms18040826
APA StyleMiyamoto, S., Komiya, M., Fujii, G., Hamoya, T., Nakanishi, R., Fujimoto, K., Tamura, S., Kurokawa, Y., Takahashi, M., Ijichi, T., & Mutoh, M. (2017). Preventive Effects of Heat-Killed Enterococcus faecalis Strain EC-12 on Mouse Intestinal Tumor Development. International Journal of Molecular Sciences, 18(4), 826. https://doi.org/10.3390/ijms18040826