Plasticizing Effects of Polyamines in Protein-Based Films
Abstract
:1. Introduction
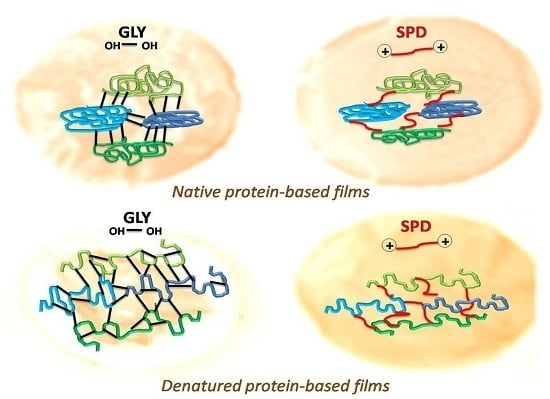
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. BV Protein Concentrate Preparation
3.3. FFS Zeta Potential and Mean Hydrodynamic Diameter Determination
3.4. BVPC Film Preparation
3.5. Differential Scanning Calorimetry Analysis
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Embuscado, M.E.; Huber, K.C. Edible Films and Coatings for Food Applications, 1st ed.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Sabbah, M.; Porta, R. Plastic pollution and the challenge of bioplastics. J. Appl. Biotechnol. Bioeng. 2017. [Google Scholar] [CrossRef]
- Hammann, F.; Schmid, M. Determination and quantification of molecular interactions in protein films: A review. Materials 2014, 7, 7975–7996. [Google Scholar] [CrossRef]
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, chemical and biochemical modifications of protein-based films and coatings: An extensive review. Int. J. Mol. Sci. 2016, 17, 1376. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Estaca, J.; Gavara, R.; Catalá, R.; Hernández-Muñoz, P. The potential of proteins for producing food packaging materials: A review. Packag. Technol. Sci. 2016, 29, 203–224. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Osako, K.; Benjakul, S.; Tongdeesoontorn, W.; Rawdkuen, S. Biodegradable protein-based films and their properties: A comparative study. Packag. Technol. Sci. 2016, 29, 77–90. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Wild, F.; Bugnicourt, E.; Cinelli, P.; Lindner, M.; Schmid, M.; Weckel, V.; Müller, K.; Rodriguez, P.; Staebler, A.; et al. State of the art in the development and properties of protein-based films and coatings and their applicability to cellulose based products: An extensive review. Coatings 2016, 6, 1. [Google Scholar] [CrossRef]
- Kokoszka, S.; Debeaufort, F.; Hambleton, A.; Lenart, A.; Voilley, A. Protein and glycerol contents affect physico-chemical properties of soy protein isolate-based edible films. Innov. Food Sci. Emerg. Technol. 2010, 11, 503–510. [Google Scholar] [CrossRef]
- Esposito, M.; Di Pierro, P.; Regalado-Gonzales, C.; Mariniello, L.; Giosafatto, C.V.L.; Porta, R. Polyamines as new cationic plasticizers for pectin-based edible films. Carbohydr. Polym. 2016, 153, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Bardocz, S.; Duguid, T.J.; Brown, D.S.; Grant, G.; Pusztai, A.; White, A.; Ralph, A. The importance of dietary polyamines in cell regeneration and growth. Br. J. Nutr. 1995, 73, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Loser, C. Polyamines in human and animal milk. Br. J. Nutr. 2000, 84, S55–S58. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, A.; Sugi, E.; Koizumi, Y.; Yanagida, F.; Udaka, S. Polyamine content of ordinary foodstuffs and various fermented foods. Biosci. Biotechnol. Biochem. 1997, 61, 1582–1584. [Google Scholar] [CrossRef] [PubMed]
- Kozova, M.; Kalac, P.; Pelikanova, T. Contents of biologically active polyamines in chicken meat, liver, heart and skin after slaughter and their changes during meat storage and cooking. Food Chem. 2009, 116, 419–425. [Google Scholar] [CrossRef]
- Nishibori, N.; Fujihara, S.; Akatuki, T. Amounts of polyamines in foods in Japan and intake by Japanese. Food Chem. 2006, 100, 491–497. [Google Scholar] [CrossRef]
- Moinard., C.; Cynober, L.; de Bandt, J.P. Polyamines: Metabolism and implications in human diseases. Clin. Nutr. 2005, 24, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Ferioli, M.E.; Pirona, L.; Pinotti, O. Prolactin and polyamine catabolism: Specific effect on polyamine oxidase activity in rat thymus. Mol. Cell. Endocrinol. 2000, 165, 51–56. [Google Scholar] [CrossRef]
- Tabor, C.W.; Tabor, H. Polyamines. Annu. Rev. Biochem. 1984, 53, 749–790. [Google Scholar] [CrossRef] [PubMed]
- Loser, C.; Folsch, U.R. Importance of various intracellular regulatory mechanisms of polyamine metabolism in camostate-induced pancreatic growth in rats. Digestion 1993, 54, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Larque, E.; Sabater-Molina, M.; Zamora, S. Biological significance of dietary polyamines. Nutrition 2007, 23, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Til, H.P.; Falke, H.E.; Prinsen, M.K.; Willems, M.I. Acute and subacute toxicity of tyramine, spermidine, spermine, putrescine and cadaverine in rats. Food Chem. Toxicol. 1997, 35, 337–348. [Google Scholar] [CrossRef]
- Polyamine. Available online: www.oryza.co.jp/html/english/pdf/polyamine_vol.2.pdf (accessed on 23 March 2017).
- Arabestani, A.; Kadivar, M.; Shahedi, M.; Goli, S.A.H.; Porta, R. Properties of a new protein film from bitter vetch (Vicia ervilia) and effect of CaCl2 on its hydropho-bicity. Int. J. Biol. Macromol. 2013, 57, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Porta, R.; Di Pierro, P.; Rossi-Marquez, G.; Mariniello, L.; Kadivar, M.; Arabestani, A. Microstructure and properties of bitter vetch (Vicia ervilia) protein films reinforced by microbial transglutaminase. Food Hydrocoll. 2015, 50, 102–107. [Google Scholar] [CrossRef]
- Arabestani, A.; Khadivar, M.; Amoresano, A.; Illiano, A.; Di Pierro, P.; Porta, R. Bitter vetch (Vicia ervilia) seed protein concentrate as possible source for production of bilayered films and biodegradable containers. Food Hydrocoll. 2016, 60, 232–242. [Google Scholar] [CrossRef]
- Sadeghi, G.H.; Mohammadi, L.; Ibrahim, S.A.; Gruber, K.J. Use of bitter vetch (Vicia ervilia) as a feed ingredient for poultry. World Poult. Sci. J. 2009, 65, 51–64. [Google Scholar] [CrossRef]
- American Association of Cereal Chemists (AACC). Approved Methods of AACC; The Association: St. Paul, MN, USA, 2003. [Google Scholar]
- Schmid, M.; Reichert, K.; Hammann, F.; Stäbler, A. Storage time-dependent alteration of molecular interaction-property relationships of whey protein isolate-based films and coatings. J. Mater. Sci. 2015, 50, 4396–4404. [Google Scholar] [CrossRef]






| Addition | Zeta Potential (mV) | ||||
|---|---|---|---|---|---|
| pH 8.0 | pH 11.0 | ||||
| −GLY | +GLY | −GLY | +GLY | ||
| A | none | −13.30 ± 0.68 | −13.00 ± 0.69 | −23.5 ± 1.01 | −20.5 ± 1.56 |
| +2 mM SPD | −10.00 ± 1.25 § | −8.48 ± 0.45 § | −22.2 ± 1.79 | −21.8 ± 0.52 | |
| +3 mM SPD | −6.68 ± 0.55 § | −5.26 ± 0.86 § | −23.5 ± 2.42 | −23.6 ± 1.34 | |
| +5 mM SPD | −3.92 ± 0.41 § | −3.57 ± 0.64 § | −22.1 ± 0.85 | −22.3 ± 1.22 | |
| +2 mM SPM | −5.09 ± 1.16 § | −6.13 ± 0.27 § | −21.1 ± 1.83 | −22.9 ± 2.76 | |
| +3 mM SPM | −3.40 ± 0.77 § | −4.00 ± 0.60 § | −23.6 ± 0.84 | −24.1 ± 2.97 | |
| +5 mM SPM | −1.80 ± 0.81 § | −2.20 ± 0.92 § | −20.5 ± 1.20 | −21.4 ± 1.70 | |
| B | none | −14.40 ± 1.72 | −13.40 ± 1.21 | −22.0 ± 1.92 | −21.9 ± 1.08 |
| +2 mM SPD | −8.13 ± 0.21 § | −11.50 ± 3.86 | −24.9 ± 3.07 | −25.9 ± 3.93 | |
| +3 mM SPD | −7.27 ± 1.83 § | −8.42 ± 0.65 § | −25.6 ± 2.98 | −24.5 ± 1.40 | |
| +5 mM SPD | −3.40 ± 0.35 § | −4.01 ± 0.93 § | −21.4 ± 2.40 | −22.2 ± 2.01 | |
| +2 mM SPM | −4.69 ± 1.09 § | −7.35 ± 0.72 § | −22.7 ± 2.29 | −26.8 ± 0.70 | |
| +3 mM SPM | −2.34 ± 0.61 § | −2.43 ± 0.74 § | −21.6 ± 0.77 | −21.2 ± 2.22 | |
| +5 mM SPM | −1.83 ± 0.49 § | −1.62 ± 0.65 § | −20.1 ± 1.50 | −20.7 ± 2.40 | |
| pH | Z-Average (d.nm) | |||||
|---|---|---|---|---|---|---|
| None | +GLY | +SPD | +SPD/GLY | +SPM | +SPM/GLY | |
| 12 | 254.3 ± 14.4 | 272.5 ± 21.7 | 273.8 ± 2.0 | 280.4 ± 4.3 | 262.9 ± 9.3 | 258.5 ± 13.1 |
| 11 | 250.1 ± 13.6 | 283.9 ± 16.2 | 272.7 ± 14.0 | 282.3 ± 17.7 | 263.0 ± 2.3 | 346.8 ± 19.2 |
| 10 | 237.5 ± 14.0 | 270.4 ± 6.3 | 298.3 ± 12.0 | 312.7 ± 12.5 | 243.0 ± 4.8 | 336.1 ± 6.9 |
| 9 | 310.1 ± 23.8 | 292.5 ± 5.7 | 319.9 ± 11.4 | 471.6 ± 65.2 | 305.7 ± 38.6 | 423.4 ± 44.8 |
| 8 | 304.3 ± 44.0 | 289.4 ± 9.6 | 477.3 ± 100.4 | 424.9 ± 55.3 | 501.8 ± 5.6 | 816.7 ± 59.5 |
| 7 | 344.2 ± 30.2 | 358.0 ± 8.4 | >1000 § | >1000 § | >1000 § | >1000 § |
| 6 | 330.5 ± 16.3 | 335.7 ± 43.2 | >1000 § | >1000 § | >1000 § | >1000 § |
| 5 | >1000 § | >1000 § | >1000 § | >1000 § | >1000 § | >1000 § |
| 4 | >1000 § | >1000 § | >1000 § | >1000 § | >1000 § | >1000 § |
| 3 | >1000 § | >1000 § | >1000 § | >1000 § | >1000 § | >1000 § |
| pH | Z-Average (d.nm) | |||||
|---|---|---|---|---|---|---|
| None | +GLY | +SPD | +SPD/GLY | +SPM | +SPM/GLY | |
| 12 | 228.9 ± 6.8 | 229.9 ± 5.4 | 242.5 ± 9.0 | 227.7 ± 5.4 | 320.0 ± 10.6 | 303.3 ± 35.5 |
| 11 | 234.5 ± 6.2 | 232.9 ± 1.3 | 229.6 ± 3.0 | 233.4 ± 3.0 | 370.8 ± 66.4 | 426.3 ± 21.6 |
| 10 | 225.4 ± 10.0 | 213.2 ± 3.3 | 210.6 ± 6.1 | 217.8 ± 3.9 | 329.0 ± 7.6 | 394.6 ± 77.2 |
| 9 | 206.6 ± 1.7 | 197.0 ± 1.4 | 187.2 ± 6.3 | 189.6 ± 10.2 | 370.7 ± 4.9 | 374.6 ± 17.0 |
| 8 | 187.9 ± 0.4 | 188.8 ± 1.3 | 227.7 ± 7.0 | 173.4 ± 3.6 | 560.6 ± 11.3 | 680.9 ± 90.0 |
| 7 | 195.4 ± 0.7 | 187.7 ± 8.7 | >1000 § | >1000 § | >1000 § | >1000 § |
| 6 | 179.4 ± 0.9 | 185.4 ± 6.0 | >1000 § | >1000 § | >1000 § | >1000 § |
| 5 | >1000 § | >1000 § | >1000 § | >1000 § | >1000 § | >1000 § |
| 4 | >1000 § | >1000 § | >1000 § | >1000 § | >1000 § | >1000 § |
| 3 | >1000 § | >1000 § | >1000 § | >1000 § | >1000 § | >1000 § |
| Components | A | B | |||
|---|---|---|---|---|---|
| GLY, | 42 mM | 100.20 ± 1.44 | 74.66 ± 1.50 | ||
| SPD, | 2 mM | 58.12 ± 2.50 § | 56.16 ± 2.87 § | ||
| 3 mM | 59.43 ± 1.81 § | 57.66 ± 1.63 § | |||
| 4 mM | 67.66 ± 1.93 § | 59.85 ± 2.51 § | |||
| 5 mM | 79.75 ± 2.68 § | 61.33 ± 1.75 § | |||
| GLY, | 25 mM + | SPD, | 2 mM + | 79.50 ± 3.56 § | 74.33 ± 2.65 |
| GLY, | 33 mM | 87.83 ± 3.25 § | 76.66 ± 2.25 | ||
| GLY, | 4 mM + | SPD, | 3 mM + | 57.50 ± 2.08 § | 56.16 ± 1.72 § |
| GLY, | 33 mM | 105.67 ± 2.84 | 75.83 ± 3.06 | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabbah, M.; Di Pierro, P.; Giosafatto, C.V.L.; Esposito, M.; Mariniello, L.; Regalado-Gonzales, C.; Porta, R. Plasticizing Effects of Polyamines in Protein-Based Films. Int. J. Mol. Sci. 2017, 18, 1026. https://doi.org/10.3390/ijms18051026
Sabbah M, Di Pierro P, Giosafatto CVL, Esposito M, Mariniello L, Regalado-Gonzales C, Porta R. Plasticizing Effects of Polyamines in Protein-Based Films. International Journal of Molecular Sciences. 2017; 18(5):1026. https://doi.org/10.3390/ijms18051026
Chicago/Turabian StyleSabbah, Mohammed, Prospero Di Pierro, C. Valeria L. Giosafatto, Marilena Esposito, Loredana Mariniello, Carlos Regalado-Gonzales, and Raffaele Porta. 2017. "Plasticizing Effects of Polyamines in Protein-Based Films" International Journal of Molecular Sciences 18, no. 5: 1026. https://doi.org/10.3390/ijms18051026
APA StyleSabbah, M., Di Pierro, P., Giosafatto, C. V. L., Esposito, M., Mariniello, L., Regalado-Gonzales, C., & Porta, R. (2017). Plasticizing Effects of Polyamines in Protein-Based Films. International Journal of Molecular Sciences, 18(5), 1026. https://doi.org/10.3390/ijms18051026