Redox Regulation of the Tumor Suppressor PTEN by Hydrogen Peroxide and Tert-Butyl Hydroperoxide
Abstract
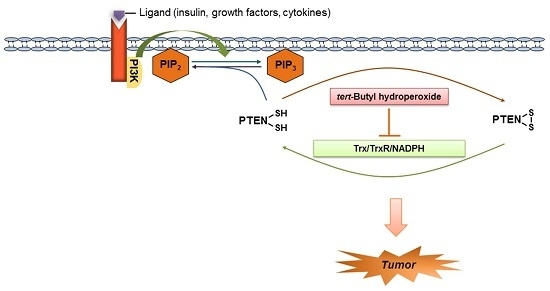
:1. Introduction
2. Results
2.1. In Vitro Oxidation of Recombinant PTEN by t-BHP
2.2. Irreversible Oxidation of PTEN in HeLa Cells Exposed to t-BHP
2.3. Exogenous Trx System Reduces Cellular PTEN Oxidized by t-BHP
2.4. t-BHP Inhibits the Reduction of Oxidized PTEN by the Trx System
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Cell Culture and Oxidation of Cellular PTEN
4.3. Oxidation of Purified PTEN
4.4. Treatment of Cell Lysates with Trx System Components
4.5. Treatment of t-BHP on the Trx System
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kubow, S. Routes of formation and toxic consequences of lipid oxidation products in foods. Free Radic. Biol. Med. 1992, 12, 63–81. [Google Scholar] [CrossRef]
- Trush, M.A.; Kensler, T.W. An overview of the relationship between oxidative stress and chemical carcinogenesis. Free Radic. Biol. Med. 1991, 10, 201–209. [Google Scholar] [CrossRef]
- Dean, R.T.; Fu, S.; Stocker, R.; Davies, M.J. Biochemistry and pathology of radical-mediated protein oxidation. Biochem. J. 1997, 324 Pt 1, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R.; Berlett, B.S. Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab. Rev. 1998, 30, 225–243. [Google Scholar] [CrossRef] [PubMed]
- Guyton, K.Z.; Gorospe, M.; Kensler, T.W.; Holbrook, N.J. Mitogen-activated protein kinase (MAPK) activation by butylated hydroxytoluene hydroperoxide: Implications for cellular survival and tumor promotion. Cancer Res. 1996, 56, 3480–3485. [Google Scholar] [PubMed]
- Kasai, H.; Kawai, K. DNA methylation at the C-5 position of cytosine by methyl radicals: A possible role for epigenetic change during carcinogenesis by environmental agents. Chem. Res. Toxicol. 2009, 22, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, P.A. Prooxidant states and tumor promotion. Science 1985, 227, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.; Guyton, K.; Egner, P.; McCarthy, T.; Lesko, S.; Akman, S. Role of reactive intermediates in tumor promotion and progression. Progr. Clin. Biol. Res. 1995, 391, 103–116. [Google Scholar]
- Taffe, B.G.; Takahashi, N.; Kensler, T.W.; Mason, R.P. Generation of free radicals from organic hydroperoxide tumor promoters in isolated mouse keratinocytes. Formation of alkyl and alkoxyl radicals from tert-butyl hydroperoxide and cumene hydroperoxide. J. Biol. Chem. 1987, 262, 12143–12149. [Google Scholar] [PubMed]
- Hix, S.; Kadiiska, M.B.; Mason, R.P.; Augusto, O. In vivo metabolism of tert-butyl hydroperoxide to methyl radicals. EPR spin-trapping and DNA methylation studies. Chem. Res. Toxicol. 2000, 13, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Norimatsu, Y.; Moriya, T.; Kobayashi, T.K.; Sakurai, T.; Shimizu, K.; Tsukayama, C.; Ohno, E. Immunohistochemical expression of PTEN and β-catenin for endometrial intraepithelial neoplasia in Japanese women. Ann. Diagn. Pathol. 2007, 11, 103–108. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Geng, X.; Zhao, P.; Fu, Z.; Wang, C.; Chao, S.; Liu, N.; Lu, A.; Gardner, K.; Pu, P.; et al. Evaluation of combination gene therapy with PTEN and antisense hTERT for malignant glioma in vitro and xenografts. Cell. Mol. Life Sci. 2007, 64, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Z.; Wang, J.; Chen, H.; Fang, J.Y. Combined PTEN mutation and protein expression associate with overall and disease-free survival of glioblastoma patients. Transl. Oncol. 2014, 7, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Schepis, C. PTEN gene: A model for genetic diseases in dermatology. Sci. World J. 2012, 2012, 252457. [Google Scholar] [CrossRef] [PubMed]
- Baig, R.M.; Mahjabeen, I.; Sabir, M.; Masood, N.; Hafeez, S.; Malik, F.A.; Kayani, M.A. Genetic changes in the PTEN gene and their association with breast cancer in Pakistan. Asian Pac. J. Cancer Prev. 2011, 12, 2773–2778. [Google Scholar] [PubMed]
- Patel, R.; Gao, M.; Ahmad, I.; Fleming, J.; Singh, L.B.; Rai, T.S.; McKie, A.B.; Seywright, M.; Barnetson, R.J.; Edwards, J.; et al. Sprouty2, PTEN, and PP2A interact to regulate prostate cancer progression. J. Clin. Investig. 2013, 123, 1157–1175. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, Y.; Nanhwan, M.K.; Ling, S.; Perez-Polo, J.R.; Ye, Y.; Bajaj, M. PTEN upregulation may explain the development of insulin resistance and type 2 diabetes with high dose statins. Cardiovasc. Drugs Ther. 2014, 28, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Worby, C.A.; Dixon, J.E. PTEN. Ann. Rev. Biochem. 2014, 83, 641–669. [Google Scholar] [CrossRef] [PubMed]
- Verrastro, I.; Tveen-Jensen, K.; Woscholski, R.; Spickett, C.M.; Pitt, A.R. Reversible oxidation of phosphatase and tensin homolog (PTEN) alters its interactions with signaling and regulatory proteins. Free Radic. Biol. Med. 2016, 90, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, E.L.; Cho, H.; Birnbaum, M.J. Role of Akt/protein kinase B in metabolism. Trends Endocrinol. Metab. 2002, 13, 444–451. [Google Scholar] [CrossRef]
- DeFeo-Jones, D.; Barnett, S.F.; Fu, S.; Hancock, P.J.; Haskell, K.M.; Leander, K.R.; McAvoy, E.; Robinson, R.G.; Duggan, M.E.; Lindsley, C.W.; et al. Tumor cell sensitization to apoptotic stimuli by selective inhibition of specific Akt/PKB family members. Mol. Cancer Ther. 2005, 4, 271–279. [Google Scholar] [PubMed]
- Myers, M.P.; Stolarov, J.P.; Eng, C.; Li, J.; Wang, S.I.; Wigler, M.H.; Parsons, R.; Tonks, N.K. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc. Natl. Acad. Sci. USA 1997, 94, 9052–9057. [Google Scholar] [CrossRef] [PubMed]
- Bermudez Brito, M.; Goulielmaki, E.; Papakonstanti, E.A. Focus on PTEN regulation. Front. Oncol. 2015, 5, 166. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.H.; Ahn, Y.; Lee, S.R.; Yeol Yeo, C.; Chung Hur, K. The major target of the endogenously generated reactive oxygen species in response to insulin stimulation is phosphatase and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase) in the PI-3 kinase/Akt pathway. Mol. Biol. Cell 2005, 16, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Boivin, B.; Zhang, S.; Arbiser, J.L.; Zhang, Z.Y.; Tonks, N.K. A modified cysteinyl-labeling assay reveals reversible oxidation of protein tyrosine phosphatases in angiomyolipoma cells. Proc. Natl. Acad. Sci. USA 2008, 105, 9959–9964. [Google Scholar] [CrossRef] [PubMed]
- Leslie, N.R.; Bennett, D.; Lindsay, Y.E.; Stewart, H.; Gray, A.; Downes, C.P. Redox regulation of PI 3-kinase signalling via inactivation of PTEN. EMBO J. 2003, 22, 5501–5510. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Yang, K.S.; Kwon, J.; Lee, C.; Jeong, W.; Rhee, S.G. Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 2002, 277, 20336–20342. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.U.; Hahne, G.; Hanske, J.; Bange, T.; Bier, D.; Rademacher, C.; Hennig, S.; Grossmann, T.N. Redox modulation of PTEN phosphatase activity by hydrogen peroxide and bisperoxidovanadium complexes. Angew. Chem. Int. Ed. 2015, 54, 13796–13800. [Google Scholar] [CrossRef] [PubMed]
- Schwertassek, U.; Haque, A.; Krishnan, N.; Greiner, R.; Weingarten, L.; Dick, T.P.; Tonks, N.K. Reactivation of oxidized PTP1B and PTEN by thioredoxin 1. FEBS J. 2014, 281, 3545–3558. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.K.; Lekli, I.; Ray, D.; Yodoi, J.; Das, D.K. Redox regulation of cell survival by the thioredoxin superfamily: An implication of redox gene therapy in the heart. Antioxid. Redox Signal. 2009, 11, 2741–2758. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, J.; Arner, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Meng, L.; Wong, J.H.; Feldman, L.J.; Lemaux, P.G.; Buchanan, B.B. A membrane-associated thioredoxin required for plant growth moves from cell to cell, suggestive of a role in intercellular communication. Proc. Natl. Acad. Sci. USA 2010, 107, 3900–3905. [Google Scholar] [CrossRef] [PubMed]
- Leslie, N.R.; Downes, C.P. PTEN: The down side of PI 3-kinase signalling. Cell. Signal. 2002, 14, 285–295. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, S.R.; Yang, K.S.; Ahn, Y.; Kim, Y.J.; Stadtman, E.R.; Rhee, S.G. Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl. Acad. Sci. USA 2004, 101, 16419–16424. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.; Sandin, A.; Forster, H.; Seiler, A.; Frijhoff, J.; Dagnell, M.; Bornkamm, G.W.; Radmark, O.; Hooft van Huijsduijnen, R.; Aspenstrom, P.; et al. 12/15-Lipoxygenase-derived lipid peroxides control receptor tyrosine kinase signaling through oxidation of protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 2010, 107, 15774–15779. [Google Scholar] [CrossRef] [PubMed]
- Weichsel, A.; Gasdaska, J.R.; Powis, G.; Montfort, W.R. Crystal structures of reduced, oxidized, and mutated human thioredoxins: Evidence for a regulatory homodimer. Structure 1996, 4, 735–751. [Google Scholar] [CrossRef]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [PubMed]
- Masaki, N.; Kyle, M.E.; Farber, J.L. tert-Butyl hydroperoxide kills cultured hepatocytes by peroxidizing membrane lipids. Arch. Biochem. Biophys. 1989, 269, 390–399. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Baysal, E.; Pashby, D.P.; Hochstein, P. t-Butyl hydroperoxide-induced perturbations of human erythrocytes as a model for oxidant stress. Biochim. Biophys. Acta 1985, 815, 426–432. [Google Scholar] [CrossRef]
- Castilho, R.F.; Kowaltowski, A.J.; Meinicke, A.R.; Bechara, E.J.; Vercesi, A.E. Permeabilization of the inner mitochondrial membrane by Ca2+ ions is stimulated by t-butyl hydroperoxide and mediated by reactive oxygen species generated by mitochondria. Free Radic. Biol. Med. 1995, 18, 479–486. [Google Scholar] [CrossRef]
- Guidarelli, A.; Clementi, E.; Brambilla, L.; Cantoni, O. Mechanism of the antimycin A-mediated enhancement of t-butylhydroperoxide-induced single-strand breakage in DNA. Biochem. J. 1997, 328 Pt 3, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Guidarelli, A.; Cattabeni, F.; Cantoni, O. Alternative mechanisms for hydroperoxide-induced DNA single strand breakage. Free Radic. Res. 1997, 26, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Lee, J.R.; Park, S.J. Role of 6-shogaol in tert-butyl hydroperoxide-induced apoptosis of HepG2 cells. Pharmacology 2014, 93, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Vanhaesebroeck, B.; Leevers, S.J.; Ahmadi, K.; Timms, J.; Katso, R.; Driscoll, P.C.; Woscholski, R.; Parker, P.J.; Waterfield, M.D. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 2001, 70, 535–602. [Google Scholar] [CrossRef] [PubMed]
- Fruman, D.A.; Meyers, R.E.; Cantley, L.C. Phosphoinositide kinases. Annu. Rev. Biochem. 1998, 67, 481–507. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Song, Y.B.; Kim, T.Y.; Kim, I.; Han, S.J.; Ahn, Y.; Cho, S.H.; Choi, C.Y.; Chay, K.O.; Yang, S.Y.; et al. Redox regulation of the tumor suppressor PTEN by glutathione. FEBS Lett. 2010, 584, 3550–3556. [Google Scholar] [CrossRef] [PubMed]
- Hashemy, S.I.; Holmgren, A. Regulation of the catalytic activity and structure of human thioredoxin 1 via oxidation and S-nitrosylation of cysteine residues. J. Biol. Chem. 2008, 283, 21890–21898. [Google Scholar] [CrossRef] [PubMed]
- Ikenoue, T.; Inoki, K.; Zhao, B.; Guan, K.L. PTEN acetylation modulates its interaction with PDZ domain. Cancer Res. 2008, 68, 6908–6912. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Bar-Noy, S.; Kwon, J.; Levine, R.L.; Stadtman, T.C.; Rhee, S.G. Mammalian thioredoxin reductase: Oxidation of the C-terminal cysteine/selenocysteine active site forms a thioselenide, and replacement of selenium with sulfur markedly reduces catalytic activity. Proc. Natl. Acad. Sci. USA 2000, 97, 2521–2526. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Ahn, Y.; Park, I.; Zhang, Y.; Kim, I.; Kim, H.W.; Ku, C.S.; Chay, K.O.; Yang, S.Y.; Ahn, B.W.; et al. Assay of the redox state of the tumor suppressor PTEN by mobility shift. Methods 2015, 77–78, 58–62. [Google Scholar] [CrossRef] [PubMed]







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Han, S.-J.; Park, I.; Kim, I.; Chay, K.-O.; Kim, S.M.; Jang, D.I.; Lee, T.-H.; Lee, S.-R. Redox Regulation of the Tumor Suppressor PTEN by Hydrogen Peroxide and Tert-Butyl Hydroperoxide. Int. J. Mol. Sci. 2017, 18, 982. https://doi.org/10.3390/ijms18050982
Zhang Y, Han S-J, Park I, Kim I, Chay K-O, Kim SM, Jang DI, Lee T-H, Lee S-R. Redox Regulation of the Tumor Suppressor PTEN by Hydrogen Peroxide and Tert-Butyl Hydroperoxide. International Journal of Molecular Sciences. 2017; 18(5):982. https://doi.org/10.3390/ijms18050982
Chicago/Turabian StyleZhang, Ying, Seong-Jeong Han, Iha Park, Inyoung Kim, Kee-Oh Chay, Seok Mo Kim, Dong Il Jang, Tae-Hoon Lee, and Seung-Rock Lee. 2017. "Redox Regulation of the Tumor Suppressor PTEN by Hydrogen Peroxide and Tert-Butyl Hydroperoxide" International Journal of Molecular Sciences 18, no. 5: 982. https://doi.org/10.3390/ijms18050982
APA StyleZhang, Y., Han, S. -J., Park, I., Kim, I., Chay, K. -O., Kim, S. M., Jang, D. I., Lee, T. -H., & Lee, S. -R. (2017). Redox Regulation of the Tumor Suppressor PTEN by Hydrogen Peroxide and Tert-Butyl Hydroperoxide. International Journal of Molecular Sciences, 18(5), 982. https://doi.org/10.3390/ijms18050982