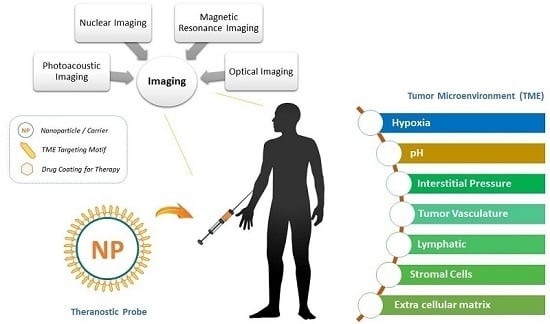
Theranostic Probes for Targeting Tumor Microenvironment: An Overview
Abstract
:1. Introduction
2. Tumor Microenvironment: Theranostic Probes for Physical Factors
2.1. Hypoxia
2.2. pH Level
2.3. Interstitial Fluid Pressure (IFP)
3. Tumor Microenvironment: Theranostic Probes for Physical Factors
3.1. Tumor Vasculature
3.2. Lymphatic System
3.3. Extracellular Matrix
3.4. Stromal Cells
4. Efficient Drug Delivery Systems
4.1. pH Based Drug Delivery System
4.2. Enzyme-Responsive Silica Based Nanomedicine
4.3. Liposome Mediated Drug
4.4. Polymeric Based Nanoparticle Theranostics
5. Challenges in Effective Cancer Theranostics
6. Conclusions
7. Future Perspective
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Swartz, M.A.; Iida, N.; Roberts, E.W.; Sangaletti, S.; Wong, M.H.; Yull, F.E.; Coussens, L.M.; DeClerck, Y.A. Tumor microenvironment complexity: Emerging roles in cancer therapy. Cancer Res. 2012, 72, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Korneev, K.V.; Atretkhany, K.-S.N.; Drutskaya, M.S.; Grivennikov, S.I.; Kuprash, D.V.; Nedospasov, S.A. TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine 2017, 89, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Spill, F.; Reynolds, D.S.; Kamm, R.D.; Zaman, M.H. Impact of the physical microenvironment on tumor progression and metastasis. Curr. Opin. Biotechnol. 2016, 40, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, W.; Li, J.; Zhang, Y. Optical imaging of tumor microenvironment. Am. J. Nucl. Med. Mol. Imaging 2013, 3, 1–15. [Google Scholar] [PubMed]
- Thiruppathi, R.; Mishra, S.; Ganapathy, M.; Padmanabhan, P.; Gulyás, B. Nanoparticle Functionalization and Its Potentials for Molecular Imaging. Adv. Sci. 2017, 4, 1600279. [Google Scholar] [CrossRef] [PubMed]
- Haigron, P.; Dillenseger, J.-L.; Luo, L.; Coatrieux, J.-L. Image-Guided Therapy: Evolution and Breakthrough. IEEE Eng. Med. Biol. Mag. 2010, 29, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.D.; Ku, S.H.; Won, Y.-Y.; Kim, S.H.; Kwon, I.C. Targeted Nanotheranostics for Future Personalized Medicine: Recent Progress in Cancer Therapy. Theranostics 2016, 6, 1362–1377. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Xie, J.; Chen, X. Peptide-based probes for targeted molecular imaging. Biochemistry 2010, 49, 1364. [Google Scholar] [CrossRef] [PubMed]
- O’Shannessy, D.J.; Somers, E.B.; Albone, E.; Cheng, X.; Park, Y.C.; Tomkowicz, B.E.; Hamuro, Y.; Kohl, T.O.; Forsyth, T.M.; Smale, R.; et al. Characterization of the human folate receptor α via novel antibody-based probes. Oncotarget 2011, 2, 1227–1243. [Google Scholar] [CrossRef] [PubMed]
- Truong, N.P.; Whittaker, M.R.; Mak, C.W.; Davis, T.P. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 2015, 12, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Qi, J.; Gogoi, R.; Wong, J.; Mitragotri, S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Control. Release 2016, 238, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Loverde, S.M.; Klein, M.L.; Discher, D.E. Nanoparticle shape improves delivery: Rational coarse grain molecular dynamics (rCG-MD) of taxol in worm-like PEG-PCL micelles. Adv. Mater. 2012, 24, 3823–3830. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Park, K.; Ryu, J.; Lee, J.J.; Lee, M.W.; Cho, H.S.; Nam, H.S.; Park, O.K.; Song, J.W.; Kim, T.S.; et al. Intravascular optical imaging of high-risk plaques in vivo by targeting macrophage mannose receptors. Sci. Rep. 2016, 6, 22608. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Dong, B.; Yan, Y.; Hu, G.; Xu, Y. Association between MMP-2 expression and prostate cancer: A meta-analysis. Biomed. Rep. 2016, 4, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.K.; Park, J.; Jon, S. Targeting Strategies for Multifunctional Nanoparticles in Cancer Imaging and Therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhuang, X.; Lin, L.; Yu, P.; Wang, Y.; Shi, Y.; Hu, G.; Sun, Y. New horizons in tumor microenvironment biology: Challenges and opportunities. BMC Med. 2015, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Thomlinson, R.H.; Gray, L.H. The Histological Structure of Some Human Lung Cancers and the Possible Implications for Radiotherapy. Br. J. Cancer 1955, 9, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Q.; Zhao, C.-L.; Li, W. Effect of hypoxia-inducible factor-1α on transcription of survivin in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2009, 28, 29. [Google Scholar] [CrossRef] [PubMed]
- Bussink, J.; Kaanders, J.H.; Rijken, P.F.; Raleigh, J.A.; Van der Kogel, A.J. Changes in Blood Perfusion and Hypoxia after Irradiation of a Human Squamous Cell Carcinoma Xenograft Tumor Line. Radiat. Res. 2000, 404, 398–404. [Google Scholar] [CrossRef]
- Erler, J.T.; Bennewith, K.L.; Nicolau, M.; Dornhöfer, N.; Kong, C.; Le, Q.-T.; Chi, J.-T.A.; Jeffrey, S.S.; Giaccia, A.J. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 2006, 440, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Michieli, P. Hypoxia, angiogenesis and cancer therapy: To breathe or not to breathe? Cell Cycle 2009, 8, 3291–3296. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G. HIF-1 Inhibitors for Cancer Therapy: From Gene Expression to Drug Discovery. Curr. Pharm. Des. 2009, 15, 3839–3843. [Google Scholar] [CrossRef] [PubMed]
- Penet, M.-F.; Krishnamachary, B.; Chen, Z.; Jin, J.; Bhujwalla, Z.M. Molecular Imaging of the Tumor Microenvironment for Precision Medicine and Theranostics. Adv. Cancer Res. 2014, 124, 235–256. [Google Scholar] [PubMed]
- Spence, A.M.; Muzi, M.; Swanson, K.R.; Sullivan, F.O.; Jason, K.; Rajendran, J.G.; Adamsen, T.C.H.; Link, J.M.; Swanson, P.E.; Yagle, K.J.; et al. Radiotherapy: Correlation with Time to Progression and Survival. Clin. Cancer Res. 2015, 14, 2623–2630. [Google Scholar] [CrossRef] [PubMed]
- Flynn, R.T.; Bowen, S.R.; Bentzen, S.M.; Mackie, T.R.; Jeraj, R. Intensity modulated X-ray (IMXT) vs. proton (IMPT) therapy for theragnostic hypoxia-based dose painting. Phys. Med. Biol. 2008, 53, 4153–4167. [Google Scholar] [CrossRef] [PubMed]
- Carlin, S.; Humm, J.L. PET of hypoxia: Current and future perspectives. J. Nucl. Med. 2012, 53, 1171–1174. [Google Scholar] [CrossRef] [PubMed]
- Mason, R.P.; Shukla, H.; Antich, P.P. In Vivo oxygen tension and temperature: Simultaneous determination using19F NMR spectroscopy of perfluorocarbon. Magn. Reson. Med. 1993, 29, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Kodibagkar, V.D.; Cui, W.; Merritt, M.E.; Mason, R.P. Novel 1H NMR approach to quantitative tissue oximetry using hexamethyldisiloxane. Magn. Reson. Med. 2006, 55, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Procissi, D.; Claus, F.; Burgman, P.; Koziorowski, J.; Chapman, J.D.; Thakur, S.B.; Matei, C.; Ling, C.C.; Koutcher, J.A. In Vivo 19F magnetic resonance spectroscopy and chemical shift imaging of tri-fluoro-nitroimidazole as a potential hypoxia reporter in solid tumors. Clin. Cancer Res. 2007, 13, 3738–3747. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.P.; Payne, G.S.; Oregioni, A.; Ruddle, R.; Tan, S.; Raynaud, F.I.; Eaton, D.; Campbell, M.J.; Cross, K.; Halbert, G.; et al. A phase I study of the nitroimidazole hypoxia marker SR4554 using 19F magnetic resonance spectroscopy. Br. J. Cancer 2009, 101, 1860–1868. [Google Scholar] [CrossRef] [PubMed]
- Gulaka, P.K.; Rojas-Quijano, F.; Kovacs, Z.; Mason, R.P.; Sherry, A.D.; Kodibagkar, V.D. GdDO3NI, a nitroimidazole-based T1 MRI contrast agent for imaging tumor hypoxia in vivo. JBIC J. Biol. Inorg. Chem. 2014, 19, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Raman, V.; Artemov, D.; Pathak, A.P.; Winnard, P.T.; McNutt, S.; Yudina, A.; Bogdanov, A.; Bhujwalla, Z.M. Characterizing vascular parameters in hypoxic regions: A combined magnetic resonance and optical imaging study of a human prostate cancer model. Cancer Res. 2006, 66, 9929–9936. [Google Scholar] [CrossRef] [PubMed]
- Kakkad, S.M.; Solaiyappan, M.; Argani, P.; Sukumar, S.; Jacobs, L.K.; Leibfritz, D.; Bhujwalla, Z.M.; Glunde, K. Collagen I fiber density increases in lymph node positive breast cancers: Pilot study. J. Biomed. Opt. 2012, 17, 116017. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Yao, J.; Wang, L.V. Photoacoustic tomography: Principles and advances. Electromagn. Waves 2014, 147, 1–22. [Google Scholar] [CrossRef]
- Liang, S.; Li, C.; Zhang, C.; Chen, Y.; Xu, L.; Bao, C.; Wang, X.; Liu, G.; Zhang, F.; Cui, D. CD44v6 monoclonal antibody-conjugated gold nanostars for targeted photoacoustic imaging and plasmonic photothermal therapy of gastric cancer stem-like cells. Theranostics 2015, 5, 970–984. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Kawamoto, K.; Izumikawa, M.; Kuriyama, H.; Yamashita, T. Gene transfer into guinea pig cochlea using adeno-associated virus vectors. J. Gene Med. 2008, 10, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Stock, C.; Schwab, A. Protons make tumor cells move like clockwork. Pflugers Arch. Eur. J. Physiol. 2009, 458, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Robey, I.; Gatenby, R.A. Causes and consequences of increased glucose metabolism of cancers. J. Nucl. Med. 2008, 49 (Suppl. S2), 24S–42S. [Google Scholar] [CrossRef] [PubMed]
- Robey, I.F.; Baggett, B.K.; Kirkpatrick, N.D.; Roe, D.J.; Dosescu, J.; Sloane, B.F.; Hashim, A.I.; Morse, D.L.; Raghunand, N.; Gatemnby, R.A.; et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 2009, 69, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Provent, P.; Benito, M.; Hiba, B.; Farion, R.; López-Larrubia, P.; Ballesteros, P.; Rémy, C.; Segebarth, C.; Cerdán, S.; Coles, J.A.; et al. Serial in vivo spectroscopic nuclear magnetic resonance imaging of lactate and extracellular pH in rat gliomas shows redistribution of protons away from sites of glycolysis. Cancer Res. 2007, 67, 7638–7645. [Google Scholar] [CrossRef] [PubMed]
- Kalash, R.; Berhane, H.; Au, J.; Rhieu, B.H.; Epperly, M.W.; Goff, J.; Dixon, T.; Wang, H.; Zhang, X.; Franicola, D.; et al. Differences in irradiated lung gene transcription between fibrosis-prone C57BL/6NHsd and fibrosis-resistant C3H/HeNHsd mice. In Vivo 2014, 28, 147–171. [Google Scholar] [PubMed]
- Moon, S.-H.; Yang, B.Y.; Kim, Y.J.; Hong, M.K.; Lee, Y.-S.; Lee, D.S.; Chung, J.-K.; Jeong, J.M. Development of a complementary PET/MR dual-modal imaging probe for targeting prostate-specific membrane antigen (PSMA). Nanomed. Nanotechnol. Biol. Med. 2016, 12, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, X.; Xie, C.; Ding, N.; Weng, X.; Lu, W.; Wei, X.; Li, C. Imaging acidosis in tumors using a pH-activated near-infrared fluorescence probe. Chem. Commun. 2012, 48, 11677–11679. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q.; Howison, C.M.; Jeffery, J.J.; Robey, I.F.; Kuo, P.H.; Pagel, M.D. Evaluations of extracellular pH within in vivo tumors using acidoCEST MRI. Magn. Reson. Med. 2014, 72, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, F.A.; Kettunen, M.I.; Day, S.E.; Hu, D.E.; Ardenkjaer-Larsen, J.H.; Zandt, R.i.; Jensen, P.R.; Karlsson, M.; Golman, K.; Lerche, M.H.; et al. Magnetic resonance imaging of pH in vivo using hyperpolarized 13C-labelled bicarbonate. Nature 2008, 453, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.H.; Li, Y.; Lee, D.S. Environmental pH-sensitive polymeric micelles for cancer diagnosis and targeted therapy. J. Control. Release 2013, 169, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Park, K.; Kim, Y.S.; Kim, M.S.; Han, J.K.; Kim, K.; Park, R.W.; Kim, I.S.; Song, H.K.; Lee, D.S.; et al. Tumoral acidic extracellular pH targeting of pH-responsive MPEG-poly(β-amino ester) block copolymer micelles for cancer therapy. J. Control. Release 2007, 123, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhang, Y.; Teng, Z.; Tian, W.; Luo, S.; Kong, X.; Su, X.; Tang, Y.; Wang, S.; Lu, G. pH-Dependent Transmembrane Activity of Peptide-functionalized Gold Nanostars for Computed Tomography/Photoacoustic Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 25, 2114–2122. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Gu, X.; Fei, Q.; Zhao, C. Photoacoustic probes for real-time tracking of endogenous H2S in living mice. Chem. Sci. 2017, 8, 2150–2155. [Google Scholar] [CrossRef]
- Siegler, E.L.; Kim, Y.J.; Wang, P. Nanomedicine targeting the tumor microenvironment: Therapeutic strategies to inhibit angiogenesis, remodel matrix, and modulate immune responses. J. Cell. Immunother. 2016, 2, 69–78. [Google Scholar] [CrossRef]
- Hompland, T.; Ellingsen, C.; Øvrebø, K.M.; Rofstad, E.K. Interstitial fluid pressure and associated lymph node metastasis revealed in tumors by dynamic contrast-enhanced MRI. Cancer Res. 2012, 72, 4899–4908. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Brown, S.L.; Ewing, J.R.; Ala, B.D.; Schneider, K.M.; Schlesinger, M. Estimation of Tumor Interstitial Fluid Pressure (TIFP) Noninvasively. PLoS ONE 2016, 11, e0140892. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Du, W.; He, B.; Fu, F.; Yuan, L.; Wu, H.; Dai, W.; Zhang, H.; Wang, X.; Wang, J.; et al. The reduction of tumor interstitial fluid pressure by liposomal imatinib and its effect on combination therapy with liposomal doxorubicin. Biomaterials 2013, 34, 2277–2288. [Google Scholar] [CrossRef] [PubMed]
- Lubberink, M.; Golla, S.S.V.; Jonasson, M.; Rubin, K.; Glimelius, B.; Sorensen, J.; Nygren, P. 15O-Water PET Study of the Effect of Imatinib, a Selective Platelet-Derived Growth Factor Receptor Inhibitor, Versus Anakinra, an IL-1R Antagonist, on Water-Perfusable Tissue Fraction in Colorectal Cancer Metastases. J. Nucl. Med. 2015, 56, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.R.; Bhojani, M.S.; McConville, P.; Moody, J.; Moffat, B.A.; Hall, D.E.; Kim, G.; Koo, Y.E.L.; Woolliscroft, M.J.; Sugai, J.V.; et al. Vascular targeted nanoparticles for imaging and treatment of brain tumors. Clin. Cancer Res. 2006, 12, 6677–6686. [Google Scholar] [CrossRef] [PubMed]
- Nanoparticles, F.M.; Enhanced, M.; Imaging, C.; Subjects, L. Fluorescent Magnetic Nanoparticles for Magnetically Enhanced Cancer Imaging and Targeting in Living. ASC Nano 2012, 6862–6869. [Google Scholar]
- Schmieder, A.H.; Caruthers, S.D.; Zhang, H.; Williams, T.A.; Robertson, J.D.; Wickline, S.A.; Lanza, G.M. Three-dimensional MR mapping of angiogenesis with α5β1(ανβ3)-targeted theranostic nanoparticles in the MDA-MB-435 xenograft mouse model. FASEB J. 2008, 22, 4179–4189. [Google Scholar] [CrossRef] [PubMed]
- Grange, C.; Geninatti-Crich, S.; Esposito, G.; Alberti, D.; Tei, L.; Bussolati, B.; Aime, S.; Camussi, G. Combined delivery and magnetic resonance imaging of neural cell adhesion molecule-targeted doxorubicin-containing liposomes in experimentally induced Kaposi’s sarcoma. Cancer Res. 2010, 70, 2180–2190. [Google Scholar] [CrossRef] [PubMed]
- Cittadino, E.; Ferraretto, M.; Torres, E.; Maiocchi, A.; Crielaard, B.J.; Lammers, T.; Storm, G.; Aime, S.; Terreno, E. MRI evaluation of the antitumor activity of paramagnetic liposomes loaded with prednisolone phosphate. Eur. J. Pharm. Sci. 2012, 45, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Collingridge, D.R.; Carroll, V.A.; Glaser, M.; Aboagye, E.O.; Osman, S.; Hutchinson, O.C.; Barthel, H.; Luthra, S.K.; Brady, F.; Bicknell, R.; et al. The development of [124I]iodinated-VG76e: A novel tracer for imaging vascular endothelial growth factor in vivo using positron emission tomography. Cancer Res. 2002, 62, 5912–5919. [Google Scholar] [PubMed]
- Ferrara, N. Vascular endothelial growth factor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Tijink, B.M.; Perk, L.R.; Budde, M.; Stigter-Van Walsum, M.; Visser, G.W.M.; Kloet, R.W.; Dinkelborg, L.M.; Leemans, C.R.; Neri, D.; Van Dongen, G.A.M.S. 124I-L19-SIP for immuno-PET imaging of tumour vasculature and guidance of 131I-L19-SIP radioimmunotherapy. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Levashova, Z.; Backer, M.; Backer, J.M.; Blankenberg, F.G. Imaging vascular endothelial growth factor (VEGF) receptors in turpentine-induced sterile thigh abscesses with radiolabeled single-chain VEGF. J. Nucl. Med. 2009, 50, 2058–2063. [Google Scholar] [CrossRef] [PubMed]
- Leung, K. Biotinylated Vascular Endothelial Growth Factor121-Avi-Streptavidin-IRDye800; Molecular Imaging and Contrast Agent Database: Bethesda, MD, USA, 2004. [Google Scholar]
- Shi, S.; Yang, K.; Hong, H.; Valdovinos, H.F.; Nayak, T.R.; Zhang, Y.; Theuer, C.P.; Barnhart, T.E.; Liu, Z.; Cai, W. Tumor vasculature targeting and imaging in living mice with reduced graphene oxide. Biomaterials 2013, 34, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Yang, K.; Hong, H.; Nickles, R.; Liu, Z.; Cai, W. In Vivo tumor vasculature targeting and imaging with VEGF-conjugated nanographene oxide. J. Nucl. Med. 2014, 55, 1371. [Google Scholar]
- Chen, F.; Nayak, T.R.; Goel, S.; Valdovinos, H.F.; Hong, H.; Theuer, C.P.; Barnhart, T.E.; Cai, W. In Vivo tumor vasculature targeted PET/NIRF imaging with TRC105 (Fab)-conjugated, dual-labeled mesoporous silica nanoparticles. Mol. Pharm. 2014, 11, 4007–4014. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chacko, A.-M.; Hu, J.; Hasegawa, K.; Swails, J.; Grasso, L.; El-Deiry, W.S.; Nicolaides, N.; Muzykantov, V.R.; Divgi, C.R.; et al. Antibody-based tumor vascular theranostics targeting endosialin/TEM1 in a new mouse tumor vascular model. Cancer Biol. Ther. 2014, 15, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ku, G.; Pageon, L.; Li, C. Theranostic probe for simultaneous in vivo photoacoustic imaging and confined photothermolysis by pulsed laser at 1064 nm in 4T1 breast cancer model. Nanoscale 2014, 6, 15228–15235. [Google Scholar] [CrossRef] [PubMed]
- Gaykema, S.B.M.; Brouwers, A.H.; Lub-de Hooge, M.N.; Pleijhuis, R.G.; Timmer-Bosscha, H.; Pot, L.; van Dam, G.M.; van der Meulen, S.B.; de Jong, J.R.; Bart, J.; et al. 89Zr-Bevacizumab PET Imaging in Primary Breast Cancer. J. Nucl. Med. 2013, 54, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Birchler, M.T.; Thuerl, C.; Schmid, D.; Neri, D.; Waibel, R.; Schubiger, A.; Stoeckli, S.J.; Schmid, S.; Goerres, G.W. Immunoscintigraphy of patients with head and neck carcinomas, with an anti-angiogenetic antibody fragment. Otolaryngol. Head Neck Surg. 2007, 136, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Shayan, R.; Achen, M.G.; Stacker, S.A. Lymphatic vessels in cancer metastasis: Bridging the gaps. Carcinogenesis 2006, 27, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Bhang, S.H.; Won, N.; Lee, T.-J.; Jin, H.; Nam, J.; Park, J.; Chung, H.; Park, H.-S.; Sung, Y.-E.; Hahn, S.K.; et al. Hyaluronic acid-quantum dot conjugates for in vivo lymphatic vessel imaging. ACS Nano 2009, 3, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Mumprecht, V.; Honer, M.; Vigl, B.; Proulx, S.T.; Trachsel, E.; Kaspar, M.; Banziger-Tobler, N.E.; Schibli, R.; Neri, D.; Detmar, M. In Vivo Imaging of inflammation-and tumor-induced lymph node lymphangiogenesis by immuno--positron emission tomography. Cancer Res. 2010, 70, 8842–8851. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Zhi, Z.; Wang, R.K. Label-free optical imaging of lymphatic vessels within tissue beds in vivo. IEEE J. Sel. Top. Quantum Electron. 2014, 20, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zou, L.G.; Zhang, S.; Gong, M.F.; Zhang, D.; Qi, Y.Y.; Zhou, S.W.; Diao, X.W. Feasibility of MR imaging in evaluating breast cancer lymphangiogenesis using Polyethylene glycol-GoldMag nanoparticles. Clin. Radiol. 2013, 68, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wu, Q.; Peng, F.; Liu, L.; Gong, C. Strategies of polymeric nanoparticles for enhanced internalization in cancer therapy. Colloids Surf. B Biointerfaces 2015, 135, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Yu, X.; Jin, C.; Yang, F.; Fu, D.; Long, J.; Xu, J.; Zhan, C.; Lu, W. LyP-1-conjugated nanoparticles for targeting drug delivery to lymphatic metastatic tumors. Int. J. Pharm. 2010, 385, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Niu, G.; Lin, X.; Jacobson, O.; Ma, Y.; Eden, H.S.; He, Y.; Lu, G.; Chen, X. Imaging tumor-induced sentinel lymph node lymphangiogenesis with LyP-1 peptide. Amino Acids 2012, 42, 2343–2351. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, Y.; Ma, J.; Zhang, H.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. LyP-1 modification to enhance delivery of artemisinin or fluorescent probe loaded polymeric micelles to highly metastatic tumor and its lymphatics. Mol. Pharm. 2012, 9, 2646–2657. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Margalit, R. Loading mitomycin C inside long circulating hyaluronan targeted nano-liposomes increases its antitumor activity in three mice tumor models. Int. J. Cancer 2004, 108, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Kanapathipillai, M.; Mammoto, A.; Mammoto, T.; Kang, J.H.; Jiang, E.; Ghosh, K.; Korin, N.; Gibbs, A.; Mannix, R.; Ingber, D.E. Inhibition of mammary tumor growth using lysyl oxidase-targeting nanoparticles to modify extracellular matrix. Nano Lett. 2012, 12, 3213–3217. [Google Scholar] [CrossRef] [PubMed]
- Yhee, J.Y.; Kim, S.A.; Koo, H.; Son, S.; Ryu, J.H.; Youn, I.C.; Choi, K.; Kim, K. Optical imaging of cancer-related proteases using near-infrared fluorescence matrix metalloproteinase-sensitive and cathepsin B-sensitive probes. Theranostics 2012, 2, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, T.; Zhang, W.; Jiang, Y.; Jin, H.; He, H.; Yang, V.C.; Chen, Y.; Huang, Y. A prodrug-type, MMP-2-targeting nanoprobe for tumor detection and imaging. Theranostics 2015, 5, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Schuerle, S.; Dudani, J.S.; Christiansen, M.G.; Anikeeva, P.; Bhatia, S.N. Magnetically Actuated Protease Sensors for in vivo Tumor Profiling. Nano Lett. 2016, 16, 6303–6310. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Foss, C.A.; Summerfield, D.D.; Doyle, J.J.; Torok, C.M.; Dietz, H.C.; Pomper, M.G.; Yu, S.M. Targeting collagen strands by photo-triggered triple-helix hybridization. Proc. Natl. Acad. Sci. USA 2012, 109, 14767–14772. [Google Scholar] [CrossRef] [PubMed]
- Heidari, P.; Esfahani, S.A.; Turker, N.S.; Wong, G.; Wang, T.C.; Rustgi, A.K.; Mahmood, U. Imaging of secreted extracellular periostin, an important marker of invasion in the tumor microenvironment in esophageal cancer. J. Nucl. Med. 2015, 56, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Keller, E.T.; Garfield, D.H.; Shen, K.; Wang, J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013, 32, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Bottazzi, B.; Colotta, F.; Sozzani, S.; Ruco, L. The origin and function of tumor-associated macrophages. Immunol. Today 1992, 13, 265–270. [Google Scholar] [CrossRef]
- Zhu, S.; Niu, M.; O’Mary, H.; Cui, Z. Targeting of tumor-associated macrophages made possible by PEG-sheddable, mannose-modified nanoparticles. Mol. Pharm. 2013, 10, 3525–3530. [Google Scholar] [CrossRef] [PubMed]
- Östman, A.; Augsten, M. Cancer-associated fibroblasts and tumor growth—Bystanders turning into key players. Curr. Opin. Genet. Dev. 2009, 19, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Brennen, W.N.; Kole, T.P.; Schneider, E.; Topaloglu, O.; Yates, M.; Cotter, R.J.; Denmeade, S.R. Fibroblast activation protein peptide substrates identified from human collagen I derived gelatin cleavage sites. Biochemistry 2008, 47, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Brennen, W.N.; Isaacs, J.T.; Denmeade, S.R. Rationale behind targeting fibroblast activation protein—Expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol. Cancer Ther. 2012, 11, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Granot, D.; Addadi, Y.; Kalchenko, V.; Harmelin, A.; Kunz-Schughart, L.A.; Neeman, M. In Vivo imaging of the systemic recruitment of fibroblasts to the angiogenic rim of ovarian carcinoma tumors. Cancer Res. 2007, 67, 9180–9189. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, M.; Krüger, J.A.; Niethammer, A.G.; Reisfeld, R.A. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J. Clin. Investig. 2006, 116, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Huang, L. Exploring the tumor microenvironment with nanoparticles. In Nanotechnology-Based Precision Tools for the Detection and Treatment of Cancer; Springer: Cham, Switzerland, 2015; pp. 193–226. [Google Scholar]
- Milner, J.M.; Patel, A.; Rowan, A.D. Emerging roles of serine proteinases in tissue turnover in arthritis. Arthritis Rheumatol. 2008, 58, 3644–3656. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.; Dong, H.; Chen, D.; Chen, W.-T. Elevation of seprase expression and promotion of an invasive phenotype by collagenous matrices in ovarian tumor cells. Int. J. Cancer 2009, 124, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Zhao, Y.; Wang, J.; Zheng, X.; Tian, Y.; Zhao, Y.; Nie, G. Tumor Fibroblast Specific Activation of a Hybrid Ferritin Nanocage-Based Optical Probe for Tumor Microenvironment Imaging. Small 2013, 9, 2427–2431. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Zhao, Y.; Ding, Y.; Wang, J.; Zhao, R.; Lang, J.; Qin, H.; Liu, X.; Shi, J.; Tao, N.; et al. Transformable Peptide Nanocarriers for Expeditious Drug Release and Effective Cancer Therapy via Cancer-Associated Fibroblast Activation. Angew. Chem. 2016, 128, 1062–1067. [Google Scholar] [CrossRef]
- Gondi, C.S.; Rao, J.S. Cathepsin B as a cancer target. Expert Opin. Ther. Targets 2013, 17, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Victor, B.C.; Anbalagan, A.; Mohamed, M.M.; Sloane, B.F.; Cavallo-Medved, D. Inhibition of cathepsin B activity attenuates extracellular matrix degradation and inflammatory breast cancer invasion. Breast Cancer Res. 2011, 13, R115. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylov, G.; Klimpel, D.; Schaschke, N.; Mikac, U.; Vizovisek, M.; Fonovic, M.; Turk, V.; Turk, B.; Vasiljeva, O. Selective targeting of tumor and stromal cells by a nanocarrier system displaying lipidated cathepsin b inhibitor. Angew. Chem. Int. Ed. 2014, 53, 10077–10081. [Google Scholar] [CrossRef] [PubMed]
- Alberto, M.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M 2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar]
- Weissleder, R.; Nahrendorf, M.; Pittet, M.J. Imaging macrophages with nanoparticles. Nat. Mater. 2014, 13, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Ries, C.H.; Cannarile, M.A.; Hoves, S.; Benz, J.; Wartha, K.; Runza, V.; Rey-Giraud, F.; Pradel, L.P.; Feuerhake, F.; Klaman, I.; et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 2014, 25, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Masaki, Y.; Shimizu, Y.; Yoshioka, T.; Tanaka, Y.; Nishijima, K.; Zhao, S.; Higashino, K.; Sakamoto, S.; Numata, Y.; Yamaguchi, Y.; et al. The accumulation mechanism of the hypoxia imaging probe “FMISO” by imaging mass spectrometry: Possible involvement of low-molecular metabolites. Sci. Rep. 2015, 5, 16802. [Google Scholar] [CrossRef] [PubMed]
- Lapi, S.E.; Lewis, J.S.; Dehdashti, F. Evaluation of hypoxia with copper-labeled diacetyl-bis (N-methylthiosemicarbazone). Semin. Nucl. Med. 2015, 45, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, M.V.; Pouremad, R.; Bloomer, W.D.; Wyrwicz, A. Novel non-invasive probes for measuring tumor-hypoxia by 19F-magnetic resonance spectroscopy (19F-MRS). Studies in the SCCVII/C3H murine model. Anticancer Res. 2006, 26, 3259–3263. [Google Scholar] [PubMed]
- Cai, Q.; Yu, T.; Zhu, W.; Xu, Y.; Qian, X. A turn-on fluorescent probe for tumor hypoxia imaging in living cells. Chem. Commun. 2015, 51, 14739–14741. [Google Scholar] [CrossRef] [PubMed]
- Van Sluis, R.; Bhujwalla, Z.M.; Raghunand, N.; Ballesteros, P.; Alvarez, J.; Cerdán, S.; Galons, J.P.; Gillies, R.J. In Vivo imaging of extracellular pH using 1H MRSI. Magn. Reson. Med. 1999, 41, 743–750. [Google Scholar] [CrossRef]
- Moon, B.F.; Jones, K.M.; Chen, L.Q.; Liu, P.; Randtke, E.A.; Howison, C.M.; Pagel, M.D. A comparison of iopromide and iopamidol, two acidoCEST MRI contrast media that measure tumor extracellular pH. Contrast Media Mol. Imaging 2015, 10, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Gao, Z.G.; Lee, E.S.; Bae, Y.H. In Vivo evaluation of doxorubicin-loaded polymeric micelles targeting folate receptors and early endosomal pH in drug-resistant ovarian cancer. Mol. Pharm. 2009, 6, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Marelli, U.K.; Rechenmacher, F.; Sobahi, T.R.A.; Mas-Moruno, C.; Kessler, H. Tumor targeting via integrin ligands. Front. Oncol. 2013, 3, 222. [Google Scholar] [CrossRef] [PubMed]
- Golestani, R.; Zeebregts, C.J.; van Scheltinga, A.G.T.T.; Hooge, M.N.L.; van Dam, G.M.; Glaudemans, A.W.J.M.; Dierckx, R.A.J.O.; Tio, R.A.; Suurmeijer, A.J.H.; Boersma, H.H.; et al. Feasibility of vascular endothelial growth factor imaging in human atherosclerotic plaque using 89Zr-bevacizumab positron emission tomography. Mol. Imaging 2013, 12, 2012–7290. [Google Scholar]
- Chen, K.; Cai, W.; Li, Z.-B.; Wang, H.; Chen, X. Quantitative PET imaging of VEGF receptor expression. Mol. Imaging Biol. 2009, 11, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Yang, K.; Hong, H.; Chen, F.; Valdovinos, H.F.; Goel, S.; Barnhart, T.E.; Liu, Z.; Cai, W. VEGFR targeting leads to significantly enhanced tumor uptake of nanographene oxide in vivo. Biomaterials 2015, 39, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.M.; Koo, H.-J.; Lee, K.C.; Choe, Y.S.; Choi, J.Y.; Lee, K.-H.; Kim, B.-T. A vascular endothelial growth factor 121 (VEGF 121)-based dual PET/optical probe for in vivo imaging of VEGF receptor expression. Biomaterials 2013, 34, 6839–6845. [Google Scholar] [CrossRef] [PubMed]
- Santimaria, M.; Moscatelli, G.; Viale, G.L.; Giovannoni, L.; Neri, G.; Viti, F.; Leprini, A.; Borsi, L.; Castellani, P.; Zardi, L.; et al. Immunoscintigraphic detection of the ED-B domain of fibronectin, a marker of angiogenesis, in patients with cancer. Clin. Cancer Res. 2003, 9, 571–579. [Google Scholar] [PubMed]
- Zhang, F.; Niu, G.; Lu, G.; Chen, X. Preclinical lymphatic imaging. Mol. Imaging Biol. 2011, 13, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Cha, E.-J.; Park, K.; Lee, S.-Y.; Hong, J.-K.; Sun, I.-C.; Kim, S.Y.; Choi, K.; Kwon, I.C.; Kim, K.; et al. A Near-Infrared-Fluorescence-Quenched Gold-Nanoparticle Imaging Probe for In Vivo Drug Screening and Protease Activity Determination. Angew. Chem. 2008, 120, 2846–2849. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.; Kim, I.K.; Park, T.G. Synthesis, characterization, and in vivo diagnostic applications of hyaluronic acid immobilized gold nanoprobes. Biomaterials 2008, 29, 4709–4718. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Foss, C.A.; Pomper, M.G.; Yu, S.M. Imaging denatured collagen strands in vivo and ex vivo via photo-triggered hybridization of caged collagen mimetic peptides. J. Vis. Exp. 2014, e51052. [Google Scholar] [CrossRef] [PubMed]
- Chopra, A. Fibroblast Activation Protein α-Specific, Near-Infrared Peptide Probe (KGPGPNQC) Linked to Cy5. 5 and a Quencher Dye, QSY21; Molecular Imaging and Contrast Agent Database: Bethesda, MD, USA, 2012. [Google Scholar]
- Wagstaff, K.; Jans, D. Protein Transduction: Cell Penetrating Peptides and Their Therapeutic Applications. Curr. Med. Chem. 2006, 13, 1371–1387. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.N.; Bu, W.; Pan, L.M.; Zhang, S.; Chen, F.; Zhou, L.; Zhao, K.L.; Peng, W.; Shi, J. Simultaneous nuclear imaging and intranuclear drug delivery by nuclear-targeted multifunctional upconversion nanoprobes. Biomaterials 2012, 33, 7282–7290. [Google Scholar] [CrossRef] [PubMed]
- Griset, A.P.; Walpole, J.; Liu, R.; Gaffey, A.; Colson, Y.L.; Grinstaff, M.W. Expansile nanoparticles: Synthesis, characterization, and in vivo efficacy of an acid-responsive polymeric drug delivery system. J. Am. Chem. Soc. 2009, 131, 2469–2471. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.T.; Ma, X.Q.; Li, C.M. Highly efficient nuclear delivery of anti-cancer drugs using a bio-functionalized reduced graphene oxide. J. Colloid Interface Sci. 2016, 467, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wang, L.; Zhang, B.; Li, D.; Meng, D.; Shi, J.; Zhang, H.; Zhang, Z.; Zhang, Y. Manganese dioxide nanosheets-based redox/pH-responsive drug delivery system for cancer theranostic application. Int. J. Nanomed. 2016, 11, 1759–1778. [Google Scholar]
- Wang, H.; Liu, G.; Dong, S.; Xiong, J.; Du, Z.; Cheng, X. A pH-responsive AIE nanoprobe as a drug delivery system for bioimaging and cancer therapy. J. Mater. Chem. B 2015, 3, 7401–7407. [Google Scholar] [CrossRef]
- Kumar, B.; Kulanthaivel, S.; Mondal, A.; Mishra, S.; Banerjee, B.; Bhaumik, A.; Banerjee, I.; Giri, S. Mesoporous silica nanoparticle based enzyme responsive system for colon specific drug delivery through guar gum capping. Colloids Surf. B Biointerfaces 2016, 150, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Aw, J.; Chen, K.; Liu, F.; Padmanabhan, P.; Hou, Y.; Cheng, Z.; Xing, B. Enzyme-Responsive Multifunctional Magnetic Nanoparticles for Tumor Intracellular Drug Delivery and Imaging. Chem. Asian J. 2011, 6, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Gao, J.; Qian, J.; Zhang, L.; Zheng, K.; Zhong, K.; Cai, D.; Zhang, X.; Wu, Z. Hydroxylated Mesoporous Nanosilica Coated by Polyethylenimine Coupled with Gadolinium and Folic Acid: A Tumor-Targeted T 1 Magnetic Resonance Contrast Agent and Drug Delivery System. ACS Appl. Mater. Interfaces 2015, 7, 14192–14200. [Google Scholar] [CrossRef] [PubMed]
- Louie, A. Multimodality imaging probes: Design and challenges. Chem. Rev. 2010, 110, 3146–3195. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.R.; Webb, M.N.; Cadotte, T.H.; Gavette, M.N. Use of targeted liposome-based chemotherapeutics to treat breast cancer. Breast Cancer Basic Clin. Res. 2015, 9, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.J.W.; Edwards, K.; Karlsson, G.; Cullis, P.R. Influence of drug-to-lipid ratio on drug release properties and liposome integrity in liposomal doxorubicin formulations. J. Liposome Res. 2008, 18, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Ali Mohammadi, Z.; Foad Aghamiri, S.; Zarrabi, A.; Reza Talaie, M. Liposomal Doxorubicin Delivery Systems: Effects of Formulation and Processing Parameters on Drug Loading and Release Behavior. Curr. Drug Deliv. 2016, 13, 1065–1070. [Google Scholar] [CrossRef]
- Chang, D.-K.; Li, P.-C.; Lu, R.-M.; Jane, W.-N.; Wu, H.-C. Peptide-mediated liposomal Doxorubicin enhances drug delivery efficiency and therapeutic efficacy in animal models. PLoS ONE 2013, 8, e83239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xiong, M.; Gong, J.; Zhang, Y.; Bai, N.; Luo, Y.; Li, L.; Wei, Y.; Liu, Y.; Tan, X.; et al. Legumain protease-activated TAT-liposome cargo for targeting tumours and their microenvironment. Nat. Commun. 2014, 5, 4280. [Google Scholar] [CrossRef] [PubMed]
- Awada, A.; Bondarenko, I.N.; Bonneterre, J.; Nowara, E.; Ferrero, J.M.; Bakshi, A.V.; Wilke, C.; Piccart, M.; Group, C.S.; et al. A randomized controlled phase II trial of a novel composition of paclitaxel embedded into neutral and cationic lipids targeting tumor endothelial cells in advanced triple-negative breast cancer (TNBC). Ann. Oncol. 2014, 25, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, I.I.; Selvakumaran, S. Designing Polymeric Nanoparticles for Targeted Drug Delivery System Outline. In Nanomedicine; One Central Press (OCP): Manchester, UK, 2014; pp. 287–313. [Google Scholar]
- Luk, B.T.; Zhang, L. Current advances in polymer-based nanotheranostics for cancer treatment and diagnosis. ACS Appl. Mater. Interfaces 2014, 6, 21859–21873. [Google Scholar] [CrossRef] [PubMed]
- Napp, J.; Behnke, T.; Fischer, L.; Würth, C.; Wottawa, M.; Katschinski, D.M.; Alves, F.; Resch-Genger, U.; Schäferling, M. Targeted luminescent near-infrared polymer-nanoprobes for in vivo imaging of tumor hypoxia. Anal. Chem. 2011, 83, 9039–9046. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.K.; Richey, J.; Strand, M.; Leslie-Pelecky, D.L.; Flask, C.A.; Labhasetwar, V. Magnetic nanoparticles with dual functional properties: Drug delivery and magnetic resonance imaging. Biomaterials 2008, 29, 4012–4021. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wang, Y.N.; Fan, Y.G.; Ma, J.B. Synthesis of a biodegradable tadpole-shaped polymer via the coupling reaction of polylactide onto mono(6-(2-aminoethyl)amino-6-deoxy)-β-cyclodextrin and its properties as the new carrier of protein delivery system. J. Control. Release 2005, 107, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Fernandez, A.; Manchanda, R.; McGoron, A.J. Theranostic Applications of Nanomaterials in Cancer: Drug Delivery, Image-Guided Therapy, and Multifunctional Platforms. Appl. Biochem. Biotechnol. 2011, 165, 1628–1651. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, O.L.; Sjaastad, K.; Radomski, M.W.; Volkov, Y.; Prina-Mello, A. Magnetic Nanoparticles in Cancer Theranostics. Theranostics 2015, 5, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Gancer 2016, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.D.; Doane, T.; Burda, C.; Basilion, J.P. Nanoparticles for imaging and treating brain cancer. Nanomedicine 2013, 8, 123–143. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, Biodistribution, and Drug-Delivery Efficiency of Mesoporous Silica Nanoparticles for Cancer Therapy in Animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Zheng, X.; Bu, W.; Ge, W.; Zhang, S.; Chen, F.; Xing, H.; Ren, Q.; Fan, W.; Zhao, K.; et al. A Core/Satellite Multifunctional Nanotheranostic for in vivo Imaging and Tumor Eradication by Radiation/Photothermal Synergistic Therapy. J. Am. Chem. Soc. 2013, 135, 13041–13048. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Katti, P.S.; Gu, Z. Enzyme-responsive nanomaterials for controlled drug delivery. Nanoscale 2014, 6, 12273–12286. [Google Scholar] [CrossRef] [PubMed]




| Serial Number | Tumor Microenvironment | Imaging Modality | Probe Design | In Vivo Model | References |
|---|---|---|---|---|---|
| 1 | Hypoxia (deoxygenated cells) | PET | 18F-FMISO | Glioblastoma patients | Spence et al. [24], Masaki et al. [108] |
| Nine-week-old male BALB/c athymic nude mice | |||||
| 2 | Hypoxia (deoxygenated cells) | PET | 61Cu-ATSM | Head and neck squamous cell carcinoma | Flynn et al. [25], Lapi et al. [109] |
| 3 | Hypoxia (deoxygenated cells) | MRS | 2-nitro-α-[(2,2,2-trifluoroethoxy) methyl]-imidazole-1-ethanol | Gastrointestinal cancer mouse model | Procissi et al. [29], Papadopoulou et al. [110] |
| BALB/c female Mice | |||||
| 4 | Hypoxia (deoxygenated cells) | MRS | GdDO3NI | Rat prostate cancer | Gulaka et al. [31] |
| 5 | Hypoxia (deoxygenated cells) | Optical Imaging | azo based fluorescent probe | Xenograft models | Kakkad et al. [33], Q. Cai et al. [111] |
| In-vitro HeLA cells | |||||
| 6 | Hypoxia | Photoacoustic imaging | CD44v6-GNS | Xenografted mouse models of gastric cancer | Liang et al. [35] |
| 7 | pH | MRS | (imidazol-1-yl)3-ethyoxycarbonylpropionic acid | Human breast cancer cells (MCF-7 and mdamb-435), grown in the mammary fat pad of severe combined immunodeficient (SCID) mice. | Van Sluis et al. [112] |
| 8 | pH | MRS | 2-(imidazol-1-yl) succinic acid | Gliomas in rat brain | Provent et al. [40] |
| 9 | pH | Optical | DilR-783-S | MDA-MB-435 xenograft model | L. Wang et al. [43] |
| 10 | pH | Optical | CEST with iopromide contrast agent | Breast cancer xenograft | Chen et al. [44], Moon et al. [113] |
| Xenograft tumor of Raji lymphoma and five mice with a xenograft tumor of MCF-7 breast cancer | |||||
| 11 | pH | Optical | doxorubicin polymeric micelles | B16F10 tumor-bearing mice | Ko et al. [47], D Kim et al. [114] |
| 4–6 week old female nude mice (BALB/c nu/nu mice) | |||||
| 12 | pH | Photoacoustic Imaging | GNS-pHLIP | Xenograft models of gastric cancer | Tian et al. [48] |
| 13 | pH | Photoacoustic Imaging | BODPA-NP | HCT116 mice cell | Shi et al. [49] |
| 14 | Interstitial Fluid Pressure | MRI | Gd-DTPA | Xenograft mouse models | Hompland et al. [51], L J. Liu et al. [52] |
| A-07 human melanoma xenografts growing in female BALB/c nu/nu mice | |||||
| 15 | Interstitial Fluid Pressure | Optical | Lysosome-Imatinib | BB16 melanoma mouse model | Fan et al. [53] |
| 16 | Tumor Vasculature | MRI | Photofrin iron oxide coated NPs | Rat glioma | Reddy et al. [55] |
| 17 | Tumor Vasculature | MRI | α5β1 RGD (Radiolabeled Arg Gly Asp) rhodamine nano particles | Α5β1 integrin in MDA-MD 435 xenograft mouse model | Schmieder et al. [57], Marelli et al. [115] |
| 18 | Tumor Vasculature | MRI | α5β1(ανβ3) fumagillin | Α5β1(ανβ3) in MDA MD 435 xenograft mouse model | Schmieder et al. [57] |
| 19 | Tumor Vasculature | MRI | NCAM targeted liposomes with doxorubicin and Gd | SCID male mice | Grange et al. [58] |
| 20 | Tumor Vasculature | MRI | PLP-LCL | B16.F10 melanoma cells injected to Male C57BI6 mice | Cittadino et al. [59] |
| 21 | Tumor Vasculature | MRI | SPIO magnetic nano particles | EGFP transfected U87MG human glioblastoma into SCID mouse | Fu Aihua et al. [56] |
| 22 | Tumor Vasculature | PET | 64Cu-NOTA-RGO-TRC105 | xenograft U87MG tumor-bearing mice | S. Shi et al. [65] |
| 23 | Tumor Vasculature | ImmunoPET | 124I MORAb-004 | Mice bearing MS1-hTEM1/fLuc or MS1/fLuc angioma grafts | Li et al. [68] |
| 24 | Tumor Vasculature | Photoacoustic imaging | PEG-CuS-NP | 4T1 breast cancer tumor bearing mice | Zhou et al. [69] |
| 25 | VEGF-A | PET | 89Zr bevacizumab | Human adenocarcinoma patients | Gaykema et al. [70], Golestani et al. [116] |
| Human carotid endarterectomy (CEA) specimens. | |||||
| 26 | VEGF-A | PET | 124I-VG67e | HT1080-26.6-bearing mice | Collingridge et al. [60] |
| 27 | VEGFR | PET | 64Cu-DOTA-VEGF121 | Mice bearing U87MG human glioblastomas | Ferrara 2009 [61], K. Chen et al. [117] |
| 28 | VEGFR | PET | NOTA-GO-VEGF121 | 4T1 murine breast tumors | Shi et al. [66], S Shi et al. [118] |
| 29 | VEGFR | SPECT | 99mTc-scVEGF | Male Swiss–Webster mice | Levashova et al. [63] |
| 30 | VEGFR | Optical | VEGF121-Avi-streptavdidn IRDye800scVEGF/Cy | Mice bearing VEGFR-2–expressing 67NR murine breast tumors | Kang et al. [119] |
| 31 | ED-B of fibronectin | PET | 124-L19-SIP | Xenograft nude mice | Tijink et al. [62] |
| 32 | Fibronectin | SPECT/CT | 123I-L19(scFv)2 | 5 male patients with head and neck cancer. | Birchler et al. [71], Santimaria et al. [120] |
| Twenty patients (34–79 years of age) with lung, colorectal, or brain cancer | |||||
| 33 | Lymphatic System | Optical | LyP-1-maleimide-PEG-PLGA-FTIC | Lymphatic metastasis tumor models, Nude BALB/c nu/nu mice | Luo et al. [78] |
| 34 | Lymphatic System | Optical | LyP-1-Cy5.5 | 4T1 murine breast cancer in mouse | Zhang et al. [79], Zhang et al. [121] |
| 35 | Lymphatic System | Optical | LyP-1-PM-ART | Nude mice bearing orthotopic MDA-MB-435S breast tumors | Z. Wang et al. [80] |
| 36 | Extracellular Matrix | Optical | HA-Au NPs-Fluorophore | C57BL/6 male mice were given a bolus injection of saline or of MMC formulations | Peer and Margalit [81], S. Lee et al. [122], H Lee et al. [123] |
| Mice bearing SCC7 tumors | |||||
| 4-Week-old DBA-1J mice | |||||
| 37 | Extracellular Matrix | Optical | LOX antibody-copolymer | Mice bearing 4T1 tumors implanted within the mammary fat pad | Kanapathipillai et al. [82] |
| 38 | Extracellular Marix | PET | 64Cu-DOTA-antiperiostin-F(ab′)2 | Genetically engineered esophageal squamous cell carcinoma mouse models | Heidari et al. [87] |
| 39 | MMP-2 | Optical | T7 peptide-LMWH-QD-LMWP-Fluorophore | Xenograft, ex vivo and in vivo of mice bearing HT1080 tumor | Y. Wang et al. [84] |
| 40 | Collagen | Optical | CMP-IR-Ahx-(GPO)9 | Prostate cancer cells were implanted subcutaneously in non-obese diabetic (NOD)/severe-combined immunodeficient (SCID) mice. | Y. Li et al. [86], Y. Li et al. [124] |
| SKH-1, DR-1 nude mice | |||||
| 41 | Stromal Cells (FAP-α) | Optical | ferritin-fluorescence peptide | Co-implants Mice bearing CAFs and PC-3 co implants | Ji et al. [100,101] |
| Fibroblast activation protein α–specific, near-infrared peptide probe (KGPGPNQC) linked to Cy5.5 and a quencher dye, QSY21 | Mice bearing C6 cell tumors (controls) or U87MG cell tumors | [125] | |||
| 42 | Stromal Cells (FAP-α) | Optical | CAP-doxorubicin-Nanoparticles | Mice bearing CAFs and PC-3 co implants | Ji et al. [100,101] |
| 43 | Stromal Cells (CtsB-PyMT tumor cells) | Optical | Doxorubicin-LNC-NS629-Gd-Alexa Fluor 555 | Mice bearing orthotopically transplanted congenic mammary tumors | Mikhaylov et al. [104] |
| 44 | Stromal Cells (CtsB-PyMT tumor cells) | Optical | mannose-PLGA-FITC | C57BL/6 miceequation | Zhu et al. [90] |
| 45 | Stromal Cells (Podoplanin) | MRI | PEG-GoldMag-nanoparticles-PodAb | Rat breast tumor model | Yang et al. [76] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikkandhar, M.G.; Nedumaran, A.M.; Ravichandar, R.; Singh, S.; Santhakumar, I.; Goh, Z.C.; Mishra, S.; Archunan, G.; Gulyás, B.; Padmanabhan, P. Theranostic Probes for Targeting Tumor Microenvironment: An Overview. Int. J. Mol. Sci. 2017, 18, 1036. https://doi.org/10.3390/ijms18051036
Sikkandhar MG, Nedumaran AM, Ravichandar R, Singh S, Santhakumar I, Goh ZC, Mishra S, Archunan G, Gulyás B, Padmanabhan P. Theranostic Probes for Targeting Tumor Microenvironment: An Overview. International Journal of Molecular Sciences. 2017; 18(5):1036. https://doi.org/10.3390/ijms18051036
Chicago/Turabian StyleSikkandhar, Musafar Gani, Anu Maashaa Nedumaran, Roopa Ravichandar, Satnam Singh, Induja Santhakumar, Zheng Cong Goh, Sachin Mishra, Govindaraju Archunan, Balázs Gulyás, and Parasuraman Padmanabhan. 2017. "Theranostic Probes for Targeting Tumor Microenvironment: An Overview" International Journal of Molecular Sciences 18, no. 5: 1036. https://doi.org/10.3390/ijms18051036
APA StyleSikkandhar, M. G., Nedumaran, A. M., Ravichandar, R., Singh, S., Santhakumar, I., Goh, Z. C., Mishra, S., Archunan, G., Gulyás, B., & Padmanabhan, P. (2017). Theranostic Probes for Targeting Tumor Microenvironment: An Overview. International Journal of Molecular Sciences, 18(5), 1036. https://doi.org/10.3390/ijms18051036