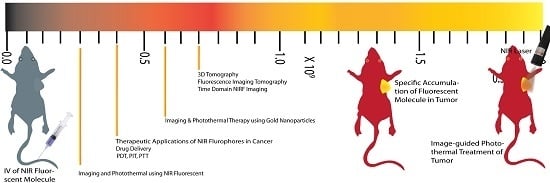
Near Infrared Fluorescence Imaging in Nano-Therapeutics and Photo-Thermal Evaluation
Abstract
:1. Introduction
2. Imaging and Photothermal Therapy Using NIR Fluorescence Molecule
2.1. The Near-Infrared Window
2.2. Requisite Design Parameters of Imaging Probes
2.3. Methods for Obtaining Tissue Specific Imaging
3. Emergent Applications of NIR Fluorescent Probes for Imaging
3.1. Nanoparticle-Based Bio-Conjugates
3.2. Small Molecule-Based Bio-Conjugates
4. Emergent Therapeutic Applications of NIR Fluorophores in Cancer Research
4.1. Drug Delivery
4.2. Photo Dynamic Therapy (PDT)
4.3. Photo Immune Therapy (PIT)
4.4. Photo Thermal Therapy (PTT)
5. Imaging and Photo-Thermal Therapy Using Gold Nanoparticles
5.1. Physical and Optical Properties of Gold Nanoparticles
5.1.1. Gold Nanostars
5.1.2. Gold Nanospheres
5.1.3. Gold Nanoshells (AuNSs)
5.1.4. Gold Nanorods
5.2. Molecular Imaging Methodologies Using NIRF Signature
6. 3D Tomography
6.1. Fluorescence Imaging Tomography
- (i)
- Depth of the tumor (axial visualization).
- (ii)
- Localization of the host organ to which the tumor is closest. By capturing the tumor illumination, it can be used to find its proximity to vital organs, which might not be visible in the planer image.
- (iii)
- The axes along which the tumor has maximum growth. This is necessary for determining the angle of injection (if intratumoral).
- (iv)
- Centre of mass (COM) of the tumor can be used for spatio-temporal simulations [74].
6.1.1. Disadvantages of FLIT Approach
6.1.2. Advances
6.2. Time Domain NIRF Imaging
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Owens, E.A.; Henary, M.; El Fakhri, G.; Choi, H.S. Tissue-specific near-infrared fluorescence imaging. Acc. Chem. Res. 2016, 49, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.; Liu, Y.; Berezin, M.Y.; Achilefu, S. Optical imaging in cancer research: Basic principles, tumor detection, and therapeutic monitoring. Med. Princ. Pract. 2011, 20, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, U.; Weissleder, R. Near-infrared optical imaging of proteases in cancer. Mol. Cancer Ther. 2003, 2, 489–496. [Google Scholar] [PubMed]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New strategies for fluorescent probe design in medical diagnostic imaging. Chem. Rev. 2009, 110, 2620–2640. [Google Scholar] [CrossRef] [PubMed]
- Owens, E.A.; Hyun, H.; Dost, T.L.; Lee, J.H.; Park, G.; Pham, D.H.; Park, M.H.; Choi, H.S.; Henary, M. Near-infrared illumination of native tissues for image-guided surgery. J. Med. Chem. 2016, 59, 5311–5323. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.; Nothdruft, R.E.; Akers, W.; Edwards, W.B.; Liang, K.; Xu, B.; Suddlow, G.P.; Deghani, H.; Tai, Y.C.; Eggebrecht, A.T.; et al. Multimodal fluorescence-mediated tomography and SPECT/CT for small-animal imaging. J. Nucl. Med. 2013, 54, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, G.; Hong, G.H.; Choi, J.; Choi, H.S. Design considerations for targeted optical contrast agents. Quant. Imaging Med. Surg. 2012, 2, 266–273. [Google Scholar] [PubMed]
- Frangioni, J.V. In vivo near-infrared fluorescence imaging. Curr. Opin. Chem. Biol. 2003, 7, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Mian, S.H.; Patel, N.A.; Farina Shah, S.B.A.; Shiekh, F.A. Research highlights from the international journal of nanomedicine 2014. Int. J. Nanomed. 2015, 10, 2503. [Google Scholar]
- Urano, Y.; Asanuma, D.; Hama, Y.; Koyama, Y.; Barrett, T.; Kamiya, M.; Nagano, T.; Watanabe, T.; Hasegawa, A.; Choyke, P.L.; et al. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat. Med. 2009, 15, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Bourne, J.R. Fractional calculus in bioengineering, part3. Crit. Rev. Biomed. Eng. 1995, 23. [Google Scholar] [CrossRef]
- Smith, B.A.; Akers, W.J.; Leevy, W.M.; Lampkins, A.J.; Xiao, S.; Wolter, W.; Suckow, M.A.; Achilefu, S.; Smith, B.D. Optical imaging of mammary and prostate tumors in living animals using a synthetic near infrared zinc (II)-dipicolylamine probe for anionic cell surfaces. J. Am. Chem. Soc. 2009, 132, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.R.; Cheng, Z.; De, A.; Rosenberg, J.; Gambhir, S.S. Dynamic visualization of RGD—Quantum dot binding to tumor neovasculature and extravasation in multiple living mouse models using intravital microscopy. Small 2010, 6, 2222–2229. [Google Scholar] [CrossRef] [PubMed]
- Van de Ven, S.M.; Elias, S.G.; Chan, C.T.; Miao, Z.; Cheng, Z.; De, A.; Gambhir, S.S. Optical imaging with Her2-targeted affibody molecules can monitor Hsp90 treatment response in a breast cancer xenograft mouse model. Clin. Cancer Res. 2012, 18, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y.C. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Gibbs, S.L.; Lee, J.H.; Kim, S.H.; Ashitate, Y.; Liu, F.; Hyun, H.; Park, G.L.; Xie, Y.; Bae, S.; et al. Targeted zwitterionic near-infrared fluorophores for improved optical imaging. Nat. Biotechnol. 2013, 31, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Mieszawska, A.J.; Kim, Y.; Gianella, A.; van Rooy, I.; Priem, B.; Labarre, M.P.; Ozcan, C.; Cormode, D.P.; Petrov, A.; Langer, R.; et al. Synthesis of polymer–lipid nanoparticles for image-guided delivery of dual modality therapy. Bioconjug. Chem. 2013, 24, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Wang, F.; Qin, W.; Yang, X.; Yuan, J. Near-infrared fluorescent probes in cancer imaging and therapy: An emerging field. Int. J. Nanomed. 2014, 9, 1347–1365. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, V.G. Photofrin-mediated photodynamic therapy for treatment of early stage oral cavity and laryngeal malignancies. Lasers Surg. Med. 2001, 29, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, R.E.; Allison, R.R.; Sibata, C.; Downie, G.H. Breast cancer with chest wall progression: Treatment with photodynamic therapy. Ann. Surg. Oncol. 2004, 11, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.R.; Huang, Z.; Korbelik, M.; Nordquist, R.E.; Liu, H. Photoimmunotherapy for cancer treatment. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Sato, K.; Hanaoka, H.; Watanabe, R.; Harada, T.; Choyke, P.L.; Kobayashi, H. The effects of conjugate and light dose on photo-immunotherapy induced cytotoxicity. BMC Cancer 2014, 14, 389. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Chen, Q.; Liu, Z. Recent advances in the development of organic photothermal nano-agents. Nano Res. 2015, 8, 340–354. [Google Scholar] [CrossRef]
- Li, Y.; Deng, Y.; Tian, X.; Ke, H.; Guo, M.; Zhu, A.; Yang, T.; Guo, Z.; Ge, Z.; Yang, X.; et al. Multipronged design of light-triggered nanoparticles to overcome cisplatin resistance for efficient ablation of resistant tumor. ACS Nano 2015, 9, 9626–9637. [Google Scholar] [CrossRef] [PubMed]
- Wan, Z.; Mao, H.; Guo, M.; Li, Y.; Zhu, A.; Yang, H.; He, H.; Shen, J.; Zhou, L.; Jiang, Z.; et al. Highly efficient hierarchical micelles integrating photothermal therapy and singlet oxygen synergized chemotherapy for cancer eradication. Theranostics 2014, 4, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.; Sershen, S.R.; Rivera, B.; Price, R.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Meneghetti, M.; Stener, M.; Guo, Y.; Chen, S.; Crespo, P.; Garcia, M.A.; Hernando, A.; Pengo, P. Pasquota. Chapter 3—Physico-Chemical Characteristics of Gold Nanoparticles. In Comprehensive Analytical Chemistry; Miguel, V., Ángela, I.L.-L., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 66, pp. 81–152. [Google Scholar]
- Quester, K.; Avalos-Borja, M.; Vilchis-Nestor, A.R.; Camacho-López, M.A. Castro-Longoria E. SERS properties of different sized and shaped gold nanoparticles biosynthesized under different environmental conditions by Neurospora crassa extract. PLoS ONE 2013, 8, e77486. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Surface-enhanced Raman scattering and biophysics. J. Phys. Condens. Matter 2002, 14, R597. [Google Scholar] [CrossRef]
- Hong, S.; Li, X. Optimal size of gold nanoparticles for surface-enhanced Raman spectroscopy under different conditions. J. Nanomater. 2013, 2013, 49. [Google Scholar] [CrossRef]
- Maier, S.A. Plasmonics: Fundamentals and Applications; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- Morandi, V.; Marabelli, F.; Amendola, V.; Meneghetti, M.; Comoretto, D. Light localization effect on the optical properties of opals doped with gold nanoparticle. J. Phys. Chem. C 2008, 112, 6293–6298. [Google Scholar] [CrossRef]
- He, H.; Xie, C.; Ren, J. Nonbleaching fluorescence of gold nanoparticles and its applications in cancer cell imaging. Anal. Chem. 2008, 80, 5951–5957. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Wang, J.; Jasinski, J.B.; Achilefu, S. Fluorescence manipulation by gold nanoparticles: From complete quenching to extensive enhancement. J. Nanobiotechnol. 2011, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, M.A. Plasmonic photo-thermal therapy (PPTT). Alex. J. Med. 2011, 47, 1–9. [Google Scholar] [CrossRef]
- Bao, Z.; Liu, X.; Liu, Y.; Liu, H.; Zhao, K. Near-infrared light-responsive inorganic nanomaterials for photothermal therapy. Asian J. Pharm. Sci. 2016, 11, 349–364. [Google Scholar] [CrossRef]
- Pitsillides, C.M.; Joe, E.K.; Wei, X.; Anderson, R.R.; Lin, C.P. Selective cell targeting with light-absorbing microparticles and nanoparticles. Biophys. J. 2003, 84, 4023–4032. [Google Scholar] [CrossRef]
- Zharov, V.P.; Mercer, K.E.; Galitovskaya, E.N.; Smeltzer, M.S. Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophys. J. 2006, 90, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, F.; Najam-ul-Haq, M.; Javeed, R.; Huck, C.W.; Bonn, G.K. Au-nanomaterials as a superior choice for near-infrared photothermal therapy. Molecules 2014, 19, 20580–20593. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gupta, S.; Li, C. Research perspectives: Gold nanoparticles in cancer theranostics. Quant. Imag. Med. Surg. 2013, 3, 284–291. [Google Scholar]
- Ali, M.R.; Wu, Y.; Han, T.; Zang, X.; Xiao, H.; Tang, Y.; Wu, R.; Fernandez, F.M.; El-Sayed, M.A. Simultaneous time-dependent surface-enhanced raman spectroscopy, metabolomics, and proteomics reveal cancer cell death mechanisms associated with gold nanorod photothermal therapy. J. Am. Chem. Soc. 2016, 138, 15434–15442. [Google Scholar] [CrossRef] [PubMed]
- Menon, J.U.; Jadeja, P.; Tambe, P.; Vu, K.; Yuan, B.; Nguyen, K.T. Nanomaterials for photo-based diagnostic and therapeutic applications. Theranostics 2013, 3, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Vendrell, M.; Maiti, K.K.; Dhaliwal, K.; Chang, Y.-T. Surface-enhanced Raman scattering in cancer detection and imaging. Trends Biotechnol. 2013, 31, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-C.; Barua, S.; Sharma, G.; Dey, S.K.; Rege, K. Inorganic nanoparticles for cancer imaging and therapy. J. Control. Release 2011, 155, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ashton, J.R.; Moding, E.J.; Yuan, H.; Register, J.K.; Fales, A.M.; Choi, J.; Whitley, M.J.; Zhao, X.; Qi, Y.; et al. A plasmonic gold nanostar theranostic probe for in vivo tumor imaging and photothermal therapy. Theranostics 2015, 5, 946. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Fu, J.; He, N.; Li, S. Advanced gold nanomaterials for photothermal therapy of cancer. J. Nanosci. Nanotechnol. 2016, 16, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, X.; Dai, S.; Ma, Y.; Cui, S.; Achilefu, S.; Gu, Y. Multifunctional Gold Nanostar Conjugates for Tumor Imaging and Combined Photothermal and Chemo-Therapy; Washington University School of Medicine: St. Louis, MO, USA, 2013. [Google Scholar]
- Li, M.; Li, L.; Zhan, C.; Kohane, D.S. Core-shell nanostars for multimodal therapy and imaging. Theranostics 2016, 6, 2306. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Chen, J.; Zhu, S.; Liu, X.; Zhou, L.; Shi, P.; Niu, D.; Gu, J.; Shi, J. Multifunctional gold nanostar-based nanocomposite: Synthesis and application for noninvasive MR-SERS imaging-guided photothermal ablation. Biomaterials 2015, 60, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold nanoparticles for biology and medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Day, E.S.; Bickford, L.R.; Slater, J.H.; Riggall, N.S.; Drezek, R.A.; West, J.L. Antibody-conjugated gold-gold sulfide nanoparticles as multifunctional agents for imaging and therapy of breast cancer. Int. J. Nanomed. 2010, 5, 445. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, J.; Huang, H.; Yu, B.; Qiu, K.; Huang, J.; Wang, S.; Jiang, L.; Gasser, G.; Ji, L.; et al. Unexpected high photothemal conversion efficiency of gold nanospheres upon grafting with two-photon luminescent ruthenium (II) complexes: A way towards cancer therapy? Biomaterials 2015, 63, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Shang, W.; Liang, X.; Liang, X.; Chen, Q.; Chi, C.; Du, Y.; Fang, C.; Tian, J. Cancer diagnosis and imaging-guided photothermal therapy using a dual-modality nanoparticle. ACS Appl. Mater. Interfaces 2016, 8, 29232–29241. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wheeler, D.; Zhang, J.Z.; Achilefu, S.; Kang, K.A. NIR fluorophore-hollow gold nanosphere complex for cancer enzyme-triggered detection and hyperthermia. In Oxygen Transport to Tissue XXXIV; Springer: Berlin, Germany, 2013; pp. 323–328. [Google Scholar]
- Rengan, A.K.; Jagtap, M.; De, A.; Banerjee, R.; Srivastava, R. Multifunctional gold coated thermo-sensitive liposomes for multimodal imaging and photo-thermal therapy of breast cancer cells. Nanoscale 2014, 6, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Rengan, A.K.; Bukhari, A.B.; Pradhan, A.; Malhotra, R.; Banerjee, R.; Srivastava, R.; De, A. In vivo analysis of biodegradable liposome gold nanoparticles as efficient agents for photothermal therapy of cancer. Nano Lett. 2015, 15, 842–848. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, D.P.; Hirsch, L.R.; Halas, N.J.; Payne, J.D.; West, J.L. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004, 209, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.; Lowery, A.; Halas, N.; West, J.; Drezek, R. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005, 5, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Topete, A.; Alatorre-Meda, M.; Iglesias, P.; Villar-Alvarez, E.M.; Barbosa, S.; Costoya, J.A.; Taboada, P.; Mosquera, V. Fluorescent drug-loaded, polymeric-based, branched gold nanoshells for localized multimodal therapy and imaging of tumoral cells. ACS Nano 2014, 8, 2725–2738. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ayala-Orozco, C.; Biswal, N.C.; Perez-Torres, C.; Bartels, M.; Bardhan, R.; Stinnet, G.; Liu, X.D.; Ji, B.; Deorukhar, A.; et al. Targeting pancreatic cancer with magneto-fluorescent theranostic gold nanoshells. Nanomedicine 2014, 9, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Nam, J.; Jung, S.; Song, J.; Doh, H.; Kim, S. Gold nanoparticle-mediated photothermal therapy: Current status and future perspective. Nanomedicine 2014, 9, 2003–2022. [Google Scholar] [CrossRef] [PubMed]
- Charan, S.; Sanjiv, K.; Singh, N.; Chien, F.-C.; Chen, Y.-F.; Nergui, N.N.; Huang, S.H.; Kuo, C.W.; Lee, T.C.; Chen, P. Development of chitosan oligosaccharide-modified gold nanorods for in vivo targeted delivery and noninvasive imaging by NIR irradiation. Bioconjug. Chem. 2012, 23, 2173–2182. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Wang, Y.; Yu, X.; Zhang, S.; Zhang, Q.; Cheng, Y. A facile strategy to prepare dendrimer-stabilized gold nanorods with sub-10-nm size for efficient photothermal cancer therapy. Sci. Rep. 2016, 6, 22764. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, M.; Zhang, J.; Zhi, X.; Li, C.; Zhang, C.; Pan, F.; Wang, K.; Yang, Y.; Fuentea, J.M.; et al. Human induced pluripotent stem cells for tumor targeted delivery of gold nanorods and enhanced photothermal therapy. ACS Nano 2016, 10, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, M.; Chen, Q.; Guan, G.; Hu, W.; Zhao, X.; Qiao, M.; Hu, H.; Liang, Y.; Zhu, H.; et al. Gold nanorods/mesoporous silica-based nanocomposite as theranostic agents for targeting near-infrared imaging and photothermal therapy induced with laser. Int. J. Nanomed. 2015, 10, 4747. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.D.; Vasquez, K.O.; Jarrell, J. Fluorescence Molecular Tomography (FMT) Imaging Techniques; PerkinElmer, Inc.: Waltham, MA, USA, 2012. [Google Scholar]
- Sevick-Muraca, E. Translation of near-infrared fluorescence imaging technologies: Emerging clinical applications. Ann. Rev. Med. 2012, 63, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Hilderbrand, S.A.; Weissleder, R. Near-infrared fluorescence: Application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 2010, 14, 71–79. [Google Scholar] [CrossRef] [PubMed]
- American Society of Mechanical Engineers. Fluorescence image reconstruction for optical tomography based on transient radiation transfer equation. In ASME 2003 International Mechanical Engineering Congress and Exposition; Quan, H., Guo, Z., Eds.; American Society of Mechanical Engineers: New York, NY, USA, 2003. [Google Scholar]
- Tucker-Schwartz, J.M.; Beavers, K.R.; Sit, W.W.; Shah, A.T.; Duvall, C.L.; Skala, M.C. In vivo imaging of nanoparticle delivery and tumor microvasculature with multimodal optical coherence tomography. Biomed. Opt. Express 2014, 5, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.Y.; Jayagopal, A. Engineering of nanoscale contrast agents for optical coherence tomography. J. Nanomed. Nanotechnol. 2014, 5, 4. [Google Scholar]
- Deliolanis, N.C.; Kasmieh, R.; Wurdinger, T.; Tannous, B.A.; Shah, K.; Ntziachristos, V. Performance of the red-shifted fluorescent proteins in deep-tissue molecular imaging applications. J. Biomed. Opt. 2008, 13, 44008. [Google Scholar] [CrossRef] [PubMed]
- Shcherbakova, D.M.; Verkhusha, V.V. Near-infrared fluorescent proteins for multicolor in vivo imaging. Nat. Methods 2013, 10, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Sano, K.; Nakajima, T.; Ali, T.; Bartlett, D.W.; Wu, A.M.; Kim, I.; Paik, C.H.; Choyke, P.L.; Kobayashi, H. Activatable fluorescent cys-diabody conjugated with indocyanine green derivative: Consideration of fluorescent catabolite kinetics on molecular imaging. J. Biomed. Opt. 2013, 18, 101304. [Google Scholar] [CrossRef] [PubMed]
- Tucker-Schwartz, J.M.; Lapierre-Landry, M.; Patil, C.A.; Skala, M.C. Photothermal optical lock-in optical coherence tomography for in vivo imaging. Biomed. Opt. Express 2015, 6, 2268–2282. [Google Scholar] [CrossRef] [PubMed]
- Antipas, V.P.; Stamatakos, G.S.; Uzunoglu, N.K.; Dionysiou, D.D.; Dale, R.G. A spatio-temporal simulation model of the response of solid tumours to radiotherapy in vivo: Parametric validation concerning oxygen enhancement ratio and cell cycle duration. Phys. Med. Biol. 2004, 49, 1485. [Google Scholar] [CrossRef] [PubMed]
- Von Maltzahn, G.; Park, J.-H.; Agrawal, A.; Bandaru, N.K.; Das, S.K.; Sailor, M.J.; Bhatia, S.N. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009, 69, 3892–3900. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-L.; Gu, M. Gold-nanoparticle-enhanced cancer photothermal therapy. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 989–996. [Google Scholar]
- Mallidi, S.; Luke, G.P.; Emelianov, S. Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance. Trends Biotechnol. 2011, 29, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Eppstein, M.J.; Hawrysz, D.J.; Godavarty, A.; Sevick-Muraca, E.M. Three-dimensional, Bayesian image reconstruction from sparse and noisy data sets: Near-infrared fluorescence tomography. Proc. Natl. Acad. Sci. USA 2002, 99, 9619–9624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, R.; Zhu, Z.; Adachi, C.; Zhang, X.; Lee, C.-S. Highly stable near-infrared fluorescent organic nanoparticles with a large stokes shift for noninvasive long-term cellular imaging. ACS Appl. Mater. Interfaces 2015, 7, 26266–26274. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef] [PubMed]
- Napp, J.; Dullin, C.; Müller, F.; Uhland, K.; Petri, J.B.; van de Locht, A.; Steinmetzer, T.; Alves, F. Time-domain in vivo near infrared fluorescence imaging for evaluation of matriptase as a potential target for the development of novel, inhibitor-based tumor therapies. Int. J. Cancer 2010, 127, 1958–1974. [Google Scholar] [CrossRef] [PubMed]
- Godavarty, A.; Eppstein, M.J.; Zhang, C.; Theru, S.; Thompson, A.B.; Gurfinkel, M.; Muraca, E.M. Fluorescence-enhanced optical imaging in large tissue volumes using a gain-modulated ICCD camera. Phys. Med. Biol. 2003, 48, 1701. [Google Scholar] [CrossRef] [PubMed]
- Optical Society of America. Hybrid PET/CT and frequency-domain based NIRF optical tomography modality for preclinical studies. In Biomedical Optics; Darne, C.D., Lu, Y., Tan, I.-C., Sevick-Muraca, E.M., Eds.; Optical Society of America: Washington, DC, USA, 2012. [Google Scholar]
- Reynolds, J.S.; Troy, T.L.; Mayer, R.H.; Thompson, A.B.; Waters, D.J.; Cornell, K.K.; Synder, P.W.; Muraca, E.M. Imaging of spontaneous canine mammary tumors using fluorescent contrast agents. Photochem. Photobiol. 1999, 70, 87–94. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vats, M.; Mishra, S.K.; Baghini, M.S.; Chauhan, D.S.; Srivastava, R.; De, A. Near Infrared Fluorescence Imaging in Nano-Therapeutics and Photo-Thermal Evaluation. Int. J. Mol. Sci. 2017, 18, 924. https://doi.org/10.3390/ijms18050924
Vats M, Mishra SK, Baghini MS, Chauhan DS, Srivastava R, De A. Near Infrared Fluorescence Imaging in Nano-Therapeutics and Photo-Thermal Evaluation. International Journal of Molecular Sciences. 2017; 18(5):924. https://doi.org/10.3390/ijms18050924
Chicago/Turabian StyleVats, Mukti, Sumit Kumar Mishra, Mahdieh Shojaei Baghini, Deepak S. Chauhan, Rohit Srivastava, and Abhijit De. 2017. "Near Infrared Fluorescence Imaging in Nano-Therapeutics and Photo-Thermal Evaluation" International Journal of Molecular Sciences 18, no. 5: 924. https://doi.org/10.3390/ijms18050924
APA StyleVats, M., Mishra, S. K., Baghini, M. S., Chauhan, D. S., Srivastava, R., & De, A. (2017). Near Infrared Fluorescence Imaging in Nano-Therapeutics and Photo-Thermal Evaluation. International Journal of Molecular Sciences, 18(5), 924. https://doi.org/10.3390/ijms18050924