Peripheral Nerve Fibers and Their Neurotransmitters in Osteoarthritis Pathology
Abstract
:1. Introduction
2. Sensory and Sympathetic Nerve Fibers in Joint Physiology
3. Sensory and Sympathetic Nerve Fibers in Osteoarthritic Joints
3.1. Sensory Nerve Fibers and Neuropeptides
3.2. Sympathetic Nerve Fibers and Neurotransmitters
4. Sensory and Sympathetic Neurotransmitters and Their Receptors in Chondrocytes
4.1. Sensory Neurotransmitters
4.2. Sympathetic Neurotransmitters
5. Sensory and Sympathetic Neurotransmitters and Their Receptors in Subchondral Bone
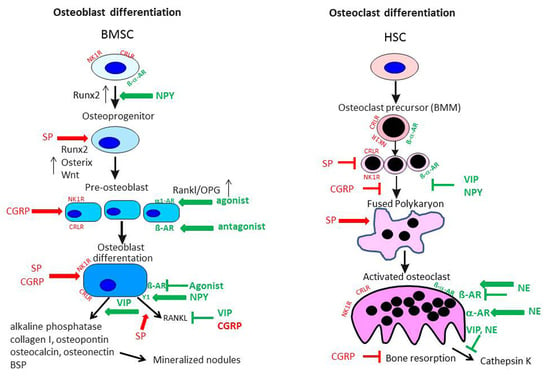
5.1. Sensory Neurotransmitters
5.1.1. Substance P Effects on Osteoblasts
5.1.2. Substance P Effects on Osteoclasts
5.1.3. CGRP Effects on Osteoblasts and Osteoclasts
5.2. Sympathetic Neurotransmitters
6. Perspectives
Acknowledgments
Conflicts of Interest
References
- Goldring, M.B.; Goldring, S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 2010, 1192, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Marcu, K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 2009, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Oliveria, S.A.; Felson, D.T.; Reed, J.I.; Cirillo, P.A.; Walker, A.M. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995, 38, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287–333. [Google Scholar] [CrossRef] [PubMed]
- Hukkanen, M.; Konttinen, Y.T.; Rees, R.G.; Santavirta, S.; Terenghi, G.; Polak, J.M. Distribution of nerve endings and sensory neuropeptides in rat synovium, meniscus and bone. Int. J. Tissue React. 1992, 14, 1–10. [Google Scholar] [PubMed]
- Madsen, J.E.; Hukkanen, M.; Aune, A.K.; Basran, I.; Moller, J.F.; Polak, J.M.; Nordsletten, L. Fracture healing and callus innervation after peripheral nerve resection in rats. Clin. Orthop. Relat Res. 1998, 351, 230–240. [Google Scholar] [CrossRef]
- Gajda, M.; Adriaensen, D.; Cichocki, T. Development of the innervation of long bones: Expression of the growth-associated protein 43. Folia Histochem. Cytobiol. 2000, 38, 103–110. [Google Scholar] [PubMed]
- Niedermair, T.; Kuhn, V.; Doranehgard, F.; Stange, R.; Wieskotter, B.; Beckmann, J.; Salmen, P.; Springorum, H.R.; Straub, R.H.; Zimmer, A.; et al. Absence of substance P and the sympathetic nervous system impact on bone structure and chondrocyte differentiation in an adult model of endochondral ossification. Matrix Biol. 2014, 38, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Grässel, S.G. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res. Ther. 2014, 16, 485. [Google Scholar] [CrossRef]
- Grässel, S.; Straub, R.H.; Jenei-Lanzl, Z. The sensory and sympathetic nervous system in cartilage physiology and pathophysiology. In Cartilage Vol. 2, Pathophysiology; Grässel, S., Aszódi, A., Eds.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Schwab, W.; Bilgicyildirim, A.; Funk, R.H. Microtopography of the autonomic nerves in the rat knee: A fluorescence microscopic study. Anat. Rec. 1997, 247, 109–118. [Google Scholar] [CrossRef]
- Schwab, W.; Funk, R.H. Innervation pattern of different cartilaginous tissues in the rat. Acta Anat. 1998, 163, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Edoff, K.; Grenegard, M.; Hildebrand, C. Retrograde tracing and neuropeptide immunohistochemistry of sensory neurones projecting to the cartilaginous distal femoral epiphysis of young rats. Cell Tissue Res. 2000, 299, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Hara-Irie, F.; Amizuka, N.; Ozawa, H. Immunohistochemical and ultrastructural localization of CGRP-positive nerve fibers at the epiphyseal trabecules facing the growth plate of rat femurs. Bone 1996, 18, 29–39. [Google Scholar] [CrossRef]
- Oliva, F.; Tarantino, U.; Maffulli, N. Immunohistochemical localization of calcitonin gene-related peptide and substance P in the rat knee cartilage at birth. Physiol. Res. 2005, 54, 549–556. [Google Scholar] [PubMed]
- Mach, D.B.; Rogers, S.D.; Sabino, M.C.; Luger, N.M.; Schwei, M.J.; Pomonis, J.D.; Keyser, C.P.; Clohisy, D.R.; Adams, D.J.; O’Leary, P.; et al. Origins of skeletal pain: Sensory and sympathetic innervation of the mouse femur. Neuroscience 2002, 113, 155–166. [Google Scholar] [CrossRef]
- Tabarowski, Z.; Gibson-Berry, K.; Felten, S.Y. Noradrenergic and peptidergic innervation of the mouse femur bone marrow. Acta Histochem. 1996, 98, 453–457. [Google Scholar] [CrossRef]
- Gajda, M.; Litwin, J.A.; Tabarowski, Z.; Zagolski, O.; Cichocki, T.; Timmermans, J.P.; Adriaensen, D. Development of rat tibia innervation: Colocalization of autonomic nerve fiber markers with growth-associated protein 43. Cells Tissues Organs 2010, 191, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Elefteriou, F. Neuronal signaling and the regulation of bone remodeling. Cell. Mol. Life Sci. 2005, 62, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Serre, C.M.; Farlay, D.; Delmas, P.D.; Chenu, C. Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone 1999, 25, 623–629. [Google Scholar] [CrossRef]
- Pagani, F.; Sibilia, V.; Cavani, F.; Ferretti, M.; Bertoni, L.; Palumbo, C.; Lattuada, N.; de Luca, E.; Rubinacci, A.; Guidobono, F. Sympathectomy alters bone architecture in adult growing rats. J. Cell. Biochem. 2008, 104, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Nifuji, A.; Takeda, S.; Ezura, Y.; Rittling, S.R.; Denhardt, D.T.; Nakashima, K.; Karsenty, G.; Noda, M. Unloading induces osteoblastic cell suppression and osteoclastic cell activation to lead to bone loss via sympathetic nervous system. J. Biol. Chem. 2005, 280, 30192–30200. [Google Scholar] [CrossRef] [PubMed]
- Togari, A.; Arai, M. Pharmacological topics of bone metabolism: The physiological function of the sympathetic nervous system in modulating bone resorption. J. Pharmacol. Sci. 2008, 106, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Suri, S.; Gill, S.E.; Massena, D.C.; Wilson, D.; McWilliams, D.F.; Walsh, D.A. Neurovascular invasion at the osteochondral junction and in osteophytes in osteoarthritis. Ann. Rheum. Dis. 2007, 66, 1423–1428. [Google Scholar] [CrossRef] [PubMed]
- Wojtys, E.M.; Beaman, D.N.; Glover, R.A.; Janda, D. Innervation of the human knee joint by substance-P fibers. Arthroscopy 1990, 6, 254–263. [Google Scholar] [CrossRef]
- Schaible, H.G. Mechanisms of chronic pain in osteoarthritis. Curr. Rheumatol. Rep. 2012, 14, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Mapp, P.I.; Avery, P.S.; McWilliams, D.F.; Bowyer, J.; Day, C.; Moores, S.; Webster, R.; Walsh, D.A. Angiogenesis in two animal models of osteoarthritis. Osteoarthr. Cartil. 2008, 16, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Mapp, P.I.; Walsh, D.A. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Pufe, T.; Petersen, W.; Tillmann, B.; Mentlein, R. The splice variants VEGF121 and VEGF189 of the angiogenic peptide vascular endothelial growth factor are expressed in osteoarthritic cartilage. Arthritis Rheum. 2001, 44, 1082–1088. [Google Scholar] [CrossRef]
- Walsh, D.A.; McWilliams, D.F.; Turley, M.J.; Dixon, M.R.; Franses, R.E.; Mapp, P.I.; Wilson, D. Angiogenesis and nerve growth factor at the osteochondral junction in rheumatoid arthritis and osteoarthritis. Rheumatology 2010, 49, 1852–1861. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Creighton-Achermann, L.; Takahashi, K.; Amiel, D.; Coutts, R.D.; Lotz, M. Development and regulation of osteophyte formation during experimental osteoarthritis. Osteoarthr. Cartil. 2002, 10, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Pfander, D.; Cramer, T.; Deuerling, D.; Weseloh, G.; Swoboda, B. Expression of thrombospondin-1 and its receptor CD36 in human osteoarthritic cartilage. Ann. Rheum. Dis. 2000, 59, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Moses, M.A.; Wiederschain, D.; Wu, I.; Fernandez, C.A.; Ghazizadeh, V.; Lane, W.S.; Flynn, E.; Sytkowski, A.; Tao, T.; Langer, R. Troponin I is present in human cartilage and inhibits angiogenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 2645–2650. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Wibberley, H.; Mapp, P.I.; Hill, R.; Wilson, D.; Walsh, D.A. Increased vascular penetration and nerve growth in the meniscus: A potential source of pain in osteoarthritis. Ann. Rheum. Dis. 2011, 70, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, M.; Wang, Q.; Izumi, M.; Tani, T. Nociceptive sensory innervation of the posterior cruciate ligament in osteoarthritic knees. Arch. Orthop. Trauma Surg. 2012, 132, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Koshino, T. Distribution of neuropeptides in synovium of the knee with osteoarthritis. Clin. Orthop. Relat. Res. 2000, 376, 172–182. [Google Scholar] [CrossRef]
- Saxler, G.; Loer, F.; Skumavc, M.; Pfortner, J.; Hanesch, U. Localization of SP- and CGRP-immunopositive nerve fibers in the hip joint of patients with painful osteoarthritis and of patients with painless failed total hip arthroplasties. Eur. J. Pain 2007, 11, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Mapp, P.I.; Walsh, D.A. Contributions of angiogenesis to inflammation, joint damage, and pain in a rat model of osteoarthritis. Arthritis Rheum. 2011, 63, 2700–2710. [Google Scholar] [CrossRef] [PubMed]
- Buma, P.; Verschuren, C.; Versleyen, D.; van der, K.P.; Oestreicher, A.B. Calcitonin gene-related peptide, substance P and GAP-43/B-50 immunoreactivity in the normal and arthrotic knee joint of the mouse. Histochemistry 1992, 98, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Nakagawa, H.; Nishimura, K.; Matsuo, S. Changes in peptidergic fiber density in the synovium of mice with collagenase-induced acute arthritis. Can. J. Physiol. Pharmacol. 2015, 93, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Saito, T. Immunohistochemical study on the effect of high tibial osteotomy on the distribution pattern of neuropeptides in the synovium of the osteoarthritic knee. Nihon Seikeigeka Gakkai Zasshi 1992, 66, 884–897. [Google Scholar] [PubMed]
- Gronblad, M.; Konttinen, Y.T.; Korkala, O.; Liesi, P.; Hukkanen, M.; Polak, J.M. Neuropeptides in synovium of patients with rheumatoid arthritis and osteoarthritis. J. Rheumatol. 1988, 15, 1807–1810. [Google Scholar] [PubMed]
- Salo, P.T.; Seeratten, R.A.; Erwin, W.M.; Bray, R.C. Evidence for a neuropathic contribution to the development of spontaneous knee osteoarthrosis in a mouse model. Acta Orthop. Scand. 2002, 73, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Chang, H.; Zhang, F.; Chen, W.; Zhu, Y.; Wu, T.; Zhang, Y. Calcitonin gene-related peptide can be selected as a predictive biomarker on progression and prognosis of knee osteoarthritis. Int. Orthop. 2015, 39, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X.; He, J.Y.; Zheng, X.F.; Li, D.; Li, Z.; Zhu, J.F.; Shen, C.; Cai, G.Q.; Chen, X.D. Increasing expression of substance P and calcitonin gene-related peptide in synovial tissue and fluid contribute to the progress of arthritis in developmental dysplasia of the hip. Arthritis Res. Ther. 2015, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Long, X.; Jiang, S.; Li, Y.; Fang, W. Histamine and substance P in synovial fluid of patients with temporomandibular disorders. J. Oral Rehabil. 2015, 42, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Justen, H.P.; Scholmerich, J.; Straub, R.H. The loss of sympathetic nerve fibers in the synovial tissue of patients with rheumatoid arthritis is accompanied by increased norepinephrine release from synovial macrophages. FASEB J. 2000, 14, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Jiao, K.; Niu, L.N.; Li, Q.H.; Ren, G.T.; Zhao, C.M.; Liu, Y.D.; Tay, F.R.; Wang, M.Q. β2-Adrenergic signal transduction plays a detrimental role in subchondral bone loss of temporomandibular joint in osteoarthritis. Sci. Rep. 2015, 5, 12593. [Google Scholar] [CrossRef] [PubMed]
- Aso, K.; Izumi, M.; Sugimura, N.; Okanoue, Y.; Ushida, T.; Ikeuchi, M. Nociceptive phenotype alterations of dorsal root ganglia neurons innervating the subchondral bone in osteoarthritic rat knee joints. Osteoarthr. Cartil. 2016, 24, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Ghilardi, J.R.; Freeman, K.T.; Jimenez-Andrade, J.M.; Coughlin, K.A.; Kaczmarska, M.J.; Castaneda-Corral, G.; Bloom, A.P.; Kuskowski, M.A.; Mantyh, P.W. Neuroplasticity of sensory and sympathetic nerve fibers in a mouse model of a painful arthritic joint. Arthritis Rheum. 2012, 64, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Eitner, A.; Pester, J.; Nietzsche, S.; Hofmann, G.O.; Schaible, H.G. The innervation of synovium of human osteoarthritic joints in comparison with normal rat and sheep synovium. Osteoarthr. Cartil. 2013, 21, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, J.; Schafer, N.; Bauer, R.; Jenei-Lanzl, Z.; Springorum, R.H.; Grassel, S. Norepinephrine modulates osteoarthritic chondrocyte metabolism and inflammatory responses. Osteoarthr. Cartil. 2016, 24, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, H.; Li, Y.S.; Luo, W. Role of vasoactive intestinal peptide in osteoarthritis. J. Biomed. Sci. 2016, 23, 63. [Google Scholar] [CrossRef] [PubMed]
- McDougall, J.J.; Watkins, L.; Li, Z. Vasoactive intestinal peptide (VIP) is a modulator of joint pain in a rat model of osteoarthritis. Pain 2006, 123, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.; Pan, H.; Peng, S.; Lv, M.; Lu, W.W. Levels of neuropeptide Y in synovial fluid relate to pain in patients with knee osteoarthritis. BMC Musculoskelet. Disord. 2014, 15, 319. [Google Scholar] [CrossRef] [PubMed]
- Opolka, A.; Straub, R.H.; Pasoldt, A.; Grifka, J.; Grassel, S. Substance P and norepinephrine modulate murine chondrocyte proliferation and apoptosis. Arthritis Rheum. 2012, 64, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Millward-Sadler, S.J.; Wright, M.O.; Davies, L.W.; Nuki, G.; Salter, D.M. Mechanotransduction via integrins and interleukin-4 results in altered aggrecan and matrix metalloproteinase 3 gene expression in normal, but not osteoarthritic, human articular chondrocytes. Arthritis Rheum. 2000, 43, 2091–2099. [Google Scholar] [CrossRef]
- Millward-Sadler, S.J.; Mackenzie, A.; Wright, M.O.; Lee, H.S.; Elliot, K.; Gerrard, L.; Fiskerstrand, C.E.; Salter, D.M.; Quinn, J.P. Tachykinin expression in cartilage and function in human articular chondrocyte mechanotransduction. Arthritis Rheum. 2003, 48, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.R.; Millward-Sadler, S.J.; Vasilliou, A.S.; Salter, D.M.; Quinn, J.P. Mechanical stimulation induces preprotachykinin gene expression in osteoarthritic chondrocytes which is correlated with modulation of the transcription factor neuron restrictive silence factor. Neuropeptides 2008, 42, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Muschter, D.; Schafer, N.; Stangl, H.; Straub, R.H.; Grassel, S. Sympathetic neurotransmitters modulate osteoclastogenesis and osteoclast activity in the context of collagen-induced arthritis. PLoS ONE 2015, 10, e0139726. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Shimoyama, Y.; Hirabayashi, K.; Kajigaya, H.; Yamamoto, S.; Oda, H.; Koshihara, Y. Production of neuropeptide substance P by synovial fibroblasts from patients with rheumatoid arthritis and osteoarthritis. Neurosci. Lett. 2001, 303, 149–152. [Google Scholar] [CrossRef]
- Im, H.J.; Li, X.; Muddasani, P.; Kim, G.H.; Davis, F.; Rangan, J.; Forsyth, C.B.; Ellman, M.; Thonas, E.J. Basic fibroblast growth factor accelerates matrix degradation via a neuro-endocrine pathway in human adult articular chondrocytes. J. Cell Physiol. 2008, 215, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M.; Carson, D.A.; Vaughan, J.H. Substance P activation of rheumatoid synoviocytes: Neural pathway in pathogenesis of arthritis. Science 1987, 235, 893–895. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Otani, H.; Mishima, K.; Ogawa, R.; Inagaki, C. Mechanisms of oxyradical production in substance P stimulated rheumatoid synovial cells. Rheumatol. Int. 1996, 16, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Lotz, M.; Vaughan, J.H.; Carson, D.A. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science 1988, 241, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Schinke, T.; Liese, S.; Priemel, M.; Haberland, M.; Schilling, A.F.; Catala-Lehnen, P.; Blicharski, D.; Rueger, J.M.; Gagel, R.F.; Emeson, R.B.; Amling, M. Decreased bone formation and osteopenia in mice lacking α-calcitonin gene-related peptide. J. Bone Miner. Res. 2004, 19, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Nakasa, T.; Ishikawa, M.; Takada, T.; Miyaki, S.; Ochi, M. Attenuation of cartilage degeneration by calcitonin gene-related paptide receptor antagonist via inhibition of subchondral bone sclerosis in osteoarthritis mice. J. Orthop. Res. 2016, 34, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Jenei-Lanzl, Z.; Grassel, S.; Pongratz, G.; Kees, F.; Miosge, N.; Angele, P.; Straub, R.H. Norepinephrine inhibits mesenchymal stem cell and chondrogenic progenitor cell chondrogenesis and accelerates chondrogenic hypertrophy. Arthritis Rheumatol. 2014, 66, 2472–2481. [Google Scholar] [CrossRef] [PubMed]
- Takarada, T.; Hojo, H.; Iemata, M.; Sahara, K.; Kodama, A.; Nakamura, N.; Hinoi, E.; Yoneda, Y. Interference by adrenaline with chondrogenic differentiation through suppression of gene transactivation mediated by Sox9 family members. Bone 2009, 45, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.P.; Mitchell, J. β2-adrenergic receptors expressed on murine chondrocytes stimulate cellular growth and inhibit the expression of Indian hedgehog and collagen type X. J. Cell. Biochem. 2008, 104, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Elefteriou, F.; Campbell, P.; Ma, Y. Control of bone remodeling by the peripheral sympathetic nervous system. Calcif. Tissue Int. 2014, 94, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, D.; Hinoi, E.; Ferron, M.; Kode, A.; Riley, K.J.; Zhou, B.; Guo, X.E.; Karsenty, G. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J. Exp. Med. 2011, 208, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, P.; SAllison, J.; Lee, N.J.; Baldock, P.A.; Brouard, N.; Rost, S.; Enriquez, R.F.; Sainsbury, A.; Lamghari, M.; Simmons, P.; et al. Greater bone formation of Y2 knockout mice is associated with increased osteoprogenitor numbers and altered Y1 receptor expression. J. Biol. Chem. 2007, 282, 19082–19091. [Google Scholar] [CrossRef] [PubMed]
- Baldock, P.A.; Sainsbury, A.; Couzens, M.; Enriquez, R.F.; Thomas, P.G.; Gardiner, E.M.; Herzog, H. Hypothalamic Y2 receptors regulate bone formation. J. Clin. Investig. 2002, 109, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, G.J. Neurohormones and catecholamines as functional components of the bone marrow microenvironment. Ann. N. Y. Acad. Sci. 2000, 917, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Maestroni, G.J.; Cosentino, M.; Marino, F.; Togni, M.; Conti, A.; Lecchini, S.; Frigo, G. Neural and endogenous catecholamines in the bone marrow. Circadian association of norepinephrine with hematopoiesis? Exp. Hematol. 1998, 26, 1172–1177. [Google Scholar] [PubMed]
- Mitchell, J.; Lai, L.P.; Peralta, F.; Xu, Y.; Sugamori, K. β2-adrenergic receptors inhibit the expression of collagen type II in growth plate chondrocytes by stimulating the AP-1 factor Jun-B. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E633–E639. [Google Scholar] [CrossRef] [PubMed]
- Poole, K.E.; Vedi, S.; Debiram, I.; Rose, C.; Power, J.; Loveridge, N.; Warburton, E.A.; Reeve, J.; Compston, J. Bone structure and remodelling in stroke patients: Early effects of zoledronate. Bone 2009, 44, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Maimoun, L.; Fattal, C.; Sultan, C. Bone remodeling and calcium homeostasis in patients with spinal cord injury: A review. Metab. Clin. Exp. 2011, 60, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.L.; Turner, R.; Elde, R. Effects of neonatal sympathectomy and capsaicin treatment on bone remodeling in rats. Neuroscience 1991, 44, 747–755. [Google Scholar] [CrossRef]
- Sherman, B.E.; Chole, R.A. In vivo effects of surgical sympathectomy on intramembranous bone resorption. Am. J. Otolaryngol. 1996, 17, 343–346. [Google Scholar] [PubMed]
- Goto, T.; Yamaza, T.; Kido, M.A.; Tanaka, T. Light- and electron-microscopic study of the distribution of axons containing substance P and the localization of neurokinin-1 receptor in bone. Cell. Tissue Res. 1998, 293, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Bjurholm, A.; Kreicbergs, A.; Schultzberg, M.; Lerner, U.H. Neuroendocrine regulation of cyclic AMP formation in osteoblastic cell lines (UMR-106-01, ROS 17/2.8, MC3T3-E1, and Saos-2) and primary bone cells. J. Bone Miner. Res. 1992, 7, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Azuma, H.; Kido, J.; Ikedo, D.; Kataoka, M.; Nagata, T. Substance P enhances the inhibition of osteoblastic cell differentiation induced by lipopolysaccharide from Porphyromonas gingivalis. J. Periodontol. 2004, 75, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Nakao, K.; Gunjigake, K.K.; Kido, M.A.; Kobayashi, S.; Tanaka, T. Substance P stimulates late-stage rat osteoblastic bone formation through neurokinin-1 receptors. Neuropeptides 2007, 41, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.B.; Chen, J.C.; Liu, Q.; Guo, M.F.; Zhang, H.P. Substance P stimulates differentiation of mice osteoblast through up-regulating Osterix expression. Chin. J. Traumatol. Zhonghua Chuang Shang Za Zhi 2010, 13, 46–50. [Google Scholar] [PubMed]
- Mei, G.; Zou, Z.; Fu, S.; Xia, L.; Zhou, J.; Zhang, Y.; Tuo, Y.; Wang, Z.; Jin, D. Substance P activates the Wnt signal transduction pathway and enhances the differentiation of mouse preosteoblastic MC3T3-E1 cells. Int. J. Mol. Sci. 2014, 15, 6224–6240. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.H.; Liu, Y.J.; Wang, W.; Zhang, Y.Z. Neuropeptide Y, substance P, and human bone morphogenetic protein 2 stimulate human osteoblast osteogenic activity by enhancing gap junction intercellular communication. Braz. J. Med. Biol. Res. 2015, 48, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Kodama, D.; Hirai, T.; Kondo, H.; Hamamura, K.; Togari, A. Bidirectional communication between sensory neurons and osteoblasts in an in vitro coculture system. FEBS Lett. 2017, 591, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, R.; Shi, X.; Wei, T.; Halloran, B.P.; Clark, D.J.; Jacobs, C.R.; Kingery, W.S. Substance P stimulates bone marrow stromal cell osteogenic activity, osteoclast differentiation, and resorption activity in vitro. Bone 2009, 45, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.J. Substance P upregulates osteoclastogenesis by activating nuclear factor κB in osteoclast precursors. Acta Otolaryngol. 2005, 125, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jeong, G.S.; Pi, S.H.; Lee, S.I.; Bae, W.J.; Kim, S.J.; Lee, S.K.; Kim, E.C. Heme oxygenase-1 protects human periodontal ligament cells against substance P-induced Rankl expression. J. Periodontal Res. 2010, 45, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Matayoshi, T.; Goto, T.; Fukuhara, E.; Takano, H.; Kobayashi, S.; Takahashi, T. Neuropeptide substance P stimulates the formation of osteoclasts via synovial fibroblastic cells. Biochem. Biophys. Res. Commun. 2005, 327, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Yu, W.; Wang, X.; Wang, B.; Chen, J.; Liu, Y.; Li, Z. Correlation between neuropeptide distribution, cancellous bone microstructure and joint pain in postmenopausal women with osteoarthritis and osteoporosis. Neuropeptides 2016, 56, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Wen, C.; Jia, X.; Li, Y.; Crane, J.L.; Mears, S.C.; Askin, F.B.; Frassica, F.J.; Chang, W.; Yao, J.; et al. Inhibition of TGF-β signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 2013, 19, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Togari, A.; Arai, M.; Mizutani, S.; Mizutani, S.; Koshihara, Y.; Nagatsu, T. Expression of mRNAs for neuropeptide receptors and β-adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci. Lett. 1997, 233, 125–128. [Google Scholar] [CrossRef]
- Uzan, B.; de Vernejoul, M.C.; Cressent, M. RAMPs and CRLR expressions in osteoblastic cells after dexamethasone treatment. Biochem. Biophys. Res. Commun. 2004, 321, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Villa, I.; Mrak, E.; Rubinacci, A.; Ravasi, F.; Guidobono, F. CGRP inhibits osteoprotegerin production in human osteoblast-like cells via cAMP/PKA-dependent pathway. Am. J. Physiol. Cell. Physiol. 2006, 291, C529–C537. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, X.; Zhao, R.; Halloran, B.P.; Clark, D.J.; Jacobs, C.R.; Kingery, W.S. Calcitonin-gene-related peptide stimulates stromal cell osteogenic differentiation and inhibits Rankl induced NF-κB activation, osteoclastogenesis and bone resorption. Bone 2010, 46, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Chai, J.; Zhang, S.; Ding, L.; Yan, P.; Du, W.; Yang, Z. CGRP may regulate bone metabolism through stimulating osteoblast differentiation and inhibiting osteoclast formation. Mol. Med. Rep. 2016, 13, 3977–3984. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.M.; Kwag, J.H.; Kim, K.H.; Kim, C.H. Effects of neuropeptides and mechanical loading on bone cell resorption in vitro. Int. J. Mol. Sci. 2014, 15, 5874–5883. [Google Scholar] [CrossRef] [PubMed]
- Lian, K.; Du, J.; Rao, Z.; Luo, H. The experimental study on the effect calcitonin gene-related peptide on bone resorption mediated by interleukin-1. J. Tongji Med. Univ. Tong Ji Yi Ke Da Xue Xue Bao 2001, 21, 304–307. [Google Scholar] [PubMed]
- Millet, I.; Vignery, A. The neuropeptide calcitonin gene-related peptide inhibits TNF-α but poorly induces IL-6 production by fetal rat osteoblasts. Cytokine 1997, 9, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Granholm, S.; Lundberg, P.; Lerner, U.H. Expression of the calcitonin receptor, calcitonin receptor-like receptor, and receptor activity modifying proteins during osteoclast differentiation. J. Cell. Biochem. 2008, 104, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.H.; Brennan, T.C.; Muir, M.M.; Mason, R.S. Functional α1- and β2-adrenergic receptors in human osteoblasts. J. Cell. Physiol. 2009, 220, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Mlakar, V.; Mlakar, S.J.; Zupan, J.; Komadina, R.; Prezelj, J.; Marc, J. ADRA2A is involved in neuro-endocrine regulation of bone resorption. J. Cell. Mol. Med. 2015, 19, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Kodama, D.; Togari, A. Noradrenaline stimulates cell proliferation by suppressing potassium channels via G(i/o) -protein-coupled α(1B)-adrenoceptors in human osteoblasts. Br. J. Pharmacol. 2013, 168, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hirai, T.; Kodama, D.; Kondo, H.; Hamamura, K.; Togari, A. α1B -Adrenoceptor signalling regulates bone formation through the up-regulation of CCAAT/enhancer-binding protein delta expression in osteoblasts. Br. J. Pharmacol. 2016, 173, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Katsumura, S.; Ezura, Y.; Izu, Y.; Shirakawa, J.; Miyawaki, A.; Harada, K.; Noda, M. β adrenergic receptor stimulation suppresses cell migration in association with cell cycle transition in osteoblasts-live imaging analyses based on FUCCI system. J. Cell. Physiol. 2016, 231, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Ezura, Y.; Hayata, T.; Moriya, S.; Shirakawa, J.; Notomi, T.; Arayal, S.; Kawasaki, M.; Izu, Y.; Harada, K.; et al. β(2) adrenergic receptor activation suppresses bone morphogenetic protein (BMP)-induced alkaline phosphatase expression in osteoblast-like MC3T3E1 cells. J. Cell. Biochem. 2015, 116, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Liang, H.; Huang, B.; Xiang, L.; Wang, T.; Xiong, Y.; Yang, B.; Guo, Y.; Gong, P. β-adrenergic signaling affect osteoclastogenesis via osteocytic MLO-Y4 cells’ Rankl production. Biochem. Biophys. Res. Commun. 2016. [Google Scholar] [CrossRef] [PubMed]
- Muschter, D.; Gottl, C.; Vogel, M.; Grifka, J.; Straub, R.H.; Grassel, S. Reactivity of rat bone marrow-derived macrophages to neurotransmitter stimulation in the context of collagen II-induced arthritis. Arthritis Res. Ther. 2015, 17, 169. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.J.; Landao-Bassonga, E.; Ralston, S.H.; Idris, A.I. β2-adrenoreceptor ligands regulate osteoclast differentiation in vitro by direct and indirect mechanisms. Arch. Biochem. Biophys. 2009, 482, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Frediani, U.; Becherini, L.; Lasagni, L.; Tanini, A.; Brandi, M.L. Catecholamines modulate growth and differentiation of human preosteoclastic cells. Osteoporos. Int. 1996, 6, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Kondo, H.; Takeuchi, S.; Togari, A. β-Adrenergic signaling stimulates osteoclastogenesis via reactive oxygen species. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E507–E515. [Google Scholar] [CrossRef] [PubMed]
- Lerner, U.H.; Persson, E. Osteotropic effects by the neuropeptides calcitonin gene-related peptide, substance P and vasoactive intestinal peptide. J. Musculoskelet. Neuronal Interact. 2008, 8, 154–165. [Google Scholar] [PubMed]
- Persson, E.; Lerner, U.H. The neuropeptide VIP regulates the expression of osteoclastogenic factors in osteoblasts. J. Cell. Biochem. 2011, 112, 3732–3741. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, T.; Helgadottir, S.L.; Tamas, A.; Reglodi, D.; Zakany, R. PACAP and VIP signaling in chondrogenesis and osteogenesis. Peptides 2015, 66, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.; Abad, C.; Martinez, C.; Leceta, J.; Gomariz, R.P. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat. Med. 2001, 7, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Horsnell, H.; Baldock, P.A. Osteoblastic Actions of the Neuropeptide Y System to Regulate Bone and Energy Homeostasis. Curr. Osteoporos. Rep. 2016, 14, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.C.; Zhang, X.B.; Hu, B.; Zi, Y.; Li, M. Neuropeptide Y accelerates post-fracture bone healing by promoting osteogenesis of mesenchymal stem cells. Neuropeptides 2016, 60, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Khor, E.C.; Yulyaningsih, E.; Driessler, F.; Kovacic, N.; Wee, N.K.; Kulkarni, R.N.; Lee, N.J.; Enriquez, R.F.; Xu, J.; Zhang, L.; Herzog, H.; Baldock, P.A. The y6 receptor suppresses bone resorption and stimulates bone formation in mice via a suprachiasmatic nucleus relay. Bone 2016, 84, 139–147. [Google Scholar] [CrossRef] [PubMed]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grässel, S.; Muschter, D. Peripheral Nerve Fibers and Their Neurotransmitters in Osteoarthritis Pathology. Int. J. Mol. Sci. 2017, 18, 931. https://doi.org/10.3390/ijms18050931
Grässel S, Muschter D. Peripheral Nerve Fibers and Their Neurotransmitters in Osteoarthritis Pathology. International Journal of Molecular Sciences. 2017; 18(5):931. https://doi.org/10.3390/ijms18050931
Chicago/Turabian StyleGrässel, Susanne, and Dominique Muschter. 2017. "Peripheral Nerve Fibers and Their Neurotransmitters in Osteoarthritis Pathology" International Journal of Molecular Sciences 18, no. 5: 931. https://doi.org/10.3390/ijms18050931
APA StyleGrässel, S., & Muschter, D. (2017). Peripheral Nerve Fibers and Their Neurotransmitters in Osteoarthritis Pathology. International Journal of Molecular Sciences, 18(5), 931. https://doi.org/10.3390/ijms18050931