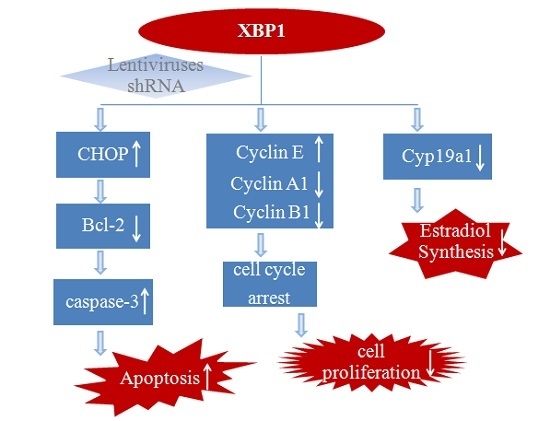
Knockdown of XBP1 by RNAi in Mouse Granulosa Cells Promotes Apoptosis, Inhibits Cell Cycle, and Decreases Estradiol Synthesis
Abstract
:1. Introduction
2. Results
2.1. XBP1 Was Efficiently Knocked Down by ShRNA-3
2.2. Knockdown of XBP1 Decreased the Concentration of E2 in Mouse GC Culture Medium
2.3. XBP1 Silencing Leads to Granulosa Cell Apoptosis
2.4. XBP1 Silencing Affects Cell Cycle and Proliferation in GCs
2.5. Silencing of XBP1 Reduced the Expression of the Key Genes in Mouse GCs
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals
4.3. Isolation and Culture of Mouse GC
4.4. Mouse GC Transduction with ShXBP1 Lentivirus
4.5. Total RNA Extraction and Real-Time PCR
4.6. Western Blot Analysis
4.7. Measurement of Estradiol and Progesterone
4.8. Cell Cycle Analysis
4.9. Cell Proliferation Assay
4.10. Cell Apoptosis Analysis
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Krentzel, A.A.; Remage-Healey, L. Sex differences and rapid estrogen signaling: A look at songbird audition. Front. Neuroendocrinol. 2015, 38, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef] [PubMed]
- Vrtacnik, P.; Ostanek, B.; Mencej-Bedrac, S.; Marc, J. The many faces of estrogen signaling. Biochem. Medica 2014, 24, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.P.; Potter, B.V. The structural biology of oestrogen metabolism. J. Steroid Biochem. Mol. Biol. 2013, 137, 27–49. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.B.; Ritter, L.J.; Armstrong, D.T. Oocyte-somatic cell interactions during follicle development in mammals. Anim. Reprod. Sci. 2004, 82, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Quirk, S.M.; Cowan, R.G.; Harman, R.M.; Hu, C.L.; Porter, D.A. Ovarian follicular growth and atresia: The relationship between cell proliferation and survival. J. Anim. Sci. 2004, 82, 40–52. [Google Scholar] [CrossRef]
- Tiwari, M.; Prasad, S.; Tripathi, A.; Pandey, A.N.; Ali, I.; Singh, A.K.; Shrivastav, T.G.; Chaube, S.K. Apoptosis in mammalian oocytes: A review. Apoptosis Int. J. Program. Cell Death 2015, 20, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Atwood, C.S.; Vadakkadath Meethal, S. The spatiotemporal hormonal orchestration of human folliculogenesis, early embryogenesis and blastocyst implantation. Mol. Cell Endocrinol. 2016, 430, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Kurata, M.; Yamazaki, Y.; Kanno, Y.; Ishibashi, S.; Takahara, T.; Kitagawa, M.; Nakamura, T. Anti-apoptotic function of XBP1 as an IL-3 signaling molecule in hematopoietic cells. Cell Death Dis. 2011, 2, e118. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, J.S. Vascular endothelial growth factor and angiogenesis: The XBP1 games. Circulation 2013, 127, 1644–1646. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Matsui, T.; Yamamoto, A.; Okada, T.; Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 2001, 107, 881–891. [Google Scholar] [CrossRef]
- Lin, J.H.; Li, H.; Yasumura, D.; Cohen, H.R.; Zhang, C.; Panning, B.; Shokat, K.M.; Lavail, M.M.; Walter, P. IRE1 signaling affects cell fate during the unfolded protein response. Science 2007, 318, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhang, Q.H.; Lu, Y.J.; Ren, K.; Yi, G.H. Involvement of the IRE1α-XBP1 pathway and XBP1S-dependent transcriptional reprogramming in metabolic diseases. DNA Cell Biol. 2015, 34, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Logue, S.E.; Cleary, P.; Saveljeva, S.; Samali, A. New directions in ER stress-induced cell death. Apoptosis Int. J. Program. Cell Death 2013, 18, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, S.J.; Koo, D.B.; Kong, I.K.; Kim, M.K.; Kime, J.M.; Choi, M.S.; Park, Y.H.; Kim, S.U.; Chang, K.T.; et al. Unfolding protein response signaling is involved in development, maintenance, and regression of the corpus luteum during the bovine estrous cycle. Biochem. Biophys. Res. Commun. 2013, 441, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.W.; Yan, J.Q.; Dou, H.T.; Liu, J.; Liu, L.; Zhao, M.L.; Liang, X.H.; Yang, Z.M. Endoplasmic reticulum stress in mouse decidua during early pregnancy. Mol. Cell. Endocrinol. 2016, 434, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Pei, X.; Jin, Y.; Wang, Y.; Zhang, C. The roles of endoplasmic reticulum stress response in female mammalian reproduction. Cell Tissue Res. 2016, 363, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yang, Y.; Li, X.; Chen, F.; Cui, C.; Hu, L.; Li, Q.; Liu, W.; Jin, Y. Endoplasmic reticulum stress is involved in granulosa cell apoptosis during follicular atresia in goat ovaries. Mol. Reprod. Dev. 2012, 79, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, P.; Chen, F.; Wang, A.; Lan, X.; Song, Y.; Jin, Y. Luman recruiting factor regulates endoplasmic reticulum stress in mouse ovarian granulosa cell apoptosis. Theriogenology 2013, 79, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, N.; Yang, D.; Wen, X.; Mahmoud, T.N.; Zhou, D.; Tang, K.; Lin, P.; Wang, A.; Jin, Y. Herp depletion arrests the S phase of the cell cycle and increases estradiol synthesis in mouse granulosa cells. J. Reprod. Dev. 2016, 62, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Diao, Y.F.; Kim, H.R.; Jin, D.I. Inhibition of endoplasmic reticulum stress improves mouse embryo development. PLoS ONE 2012, 7, e40433. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Iliopoulos, D.; Zhang, Q.; Tang, Q.; Greenblatt, M.B.; Hatziapostolou, M.; Lim, E.; Tam, W.L.; Ni, M.; Chen, Y.; et al. XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway. Nature 2014, 508, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yan, J.; Zhu, J.; Zhong, H.; Lu, Q.; Wang, Z.; Huang, C.; Ye, Q. Ligand-independent activation of estrogen receptor alpha by XBP1. Nucleic Acids Res. 2003, 31, 5266–5274. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, S.J.; Kim, T.S.; Kim, J.M.; Lee, D.S. Testosterone production by a leydig tumor cell line is suppressed by hyperthermia-induced endoplasmic reticulum stress in mice. Life Sci. 2016, 146, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, L.; Lin, P.; Jiang, T.; Wang, N.; Zhao, F.; Chen, H.; Tang, K.; Zhou, D.; Wang, A.; et al. An immortalized steroidogenic goat granulosa cell line as a model system to study the effect of the endoplasmic reticulum (ER)-stress response on steroidogenesis. J. Reprod. Dev. 2017, 63, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, F.; Wang, N.; Yi, Y.; Lin, P.; Tang, K.; Wang, A.; Jin, Y. Knockdown of CREB3/luman by shRNA in mouse granulosa cells results in decreased estradiol and progesterone synthesis and promotes cell proliferation. PLoS ONE 2016, 11, e0168246. [Google Scholar]
- Xiong, Y.; Chen, H.; Lin, P.; Wang, A.; Wang, L.; Jin, Y. ATF6 knockdown decreases apoptosis, arrests the S phase of the cell cycle, and increases steroid hormone production in mouse granulosa cells. Am. J. Physiol. Cell Physiol. 2017, 312, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Nose, E.; Takahashi, N.; Hirota, Y.; Hirata, T.; Yoshino, O.; Koga, K.; Fujii, T.; Osuga, Y. Evidence of the activation of unfolded protein response in granulosa and cumulus cells during follicular growth and maturation. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2015, 31, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Albertini, D.F. A cell for every season: The ovarian granulosa cell. J. Assist. Reprod. Genet. 2011, 28, 877–878. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Yu, Y.; Qiao, J. Dual role for the unfolded protein response in the ovary: Adaption and apoptosis. Protein Cell 2017, 8, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Erickson, G.F.; Danforth, D.R. Ovarian control of follicle development. Am. J. Obstet. Gynecol. 1995, 172, 736–747. [Google Scholar] [CrossRef]
- Chaube, S.K.; Prasad, P.V.; Thakur, S.C.; Shrivastav, T.G. Estradiol protects clomiphene citrate-induced apoptosis in ovarian follicular cells and ovulated cumulus-oocyte complexes. Fertil. Steril. 2005, 84, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Shrivastav, T.G.; Chaube, S.K. An increase of granulosa cell apoptosis mediates aqueous neem (Azadirachta indica) leaf extract-induced oocyte apoptosis in rat. Int. J. Appl. Basic Med. Res. 2013, 3, 27–36. [Google Scholar] [PubMed]
- Yang, D.; Jiang, T.; Lin, P.; Chen, H.; Wang, L.; Wang, N.; Zhao, F.; Wang, A.; Jin, Y. Knock-down of apoptosis inducing factor gene protects endoplasmic reticulum stress-mediated goat granulosa cell apoptosis. Theriogenology 2017, 88, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Bahar, E.; Kim, H.; Yoon, H. ER stress-mediated signaling: Action potential and Ca2+ as key players. Int. J. Mol. Sci. 2016, 17, 1558. [Google Scholar] [CrossRef] [PubMed]
- Puthalakath, H.; O’Reilly, L.A.; Gunn, P.; Lee, L.; Kelly, P.N.; Huntington, N.D.; Hughes, P.D.; Michalak, E.M.; McKimm-Breschkin, J.; Motoyama, N.; et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 2007, 129, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Martinou, J.C.; Youle, R.J. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev. Cell 2011, 21, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.U.; Kornfeld, O.S.; Mochly-Rosen, D. The entangled ER-mitochondrial axis as a potential therapeutic strategy in neurodegeneration: A tangled duo unchained. Cell Calcium 2016, 60, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, E.; Ergin, K.; Basaloglu, H.; Koca, Y.; Seyrek, K. Expression and localisation of Bcl-2 and bax proteins in developing rat ovary. Res. Vet. Sci. 2008, 84, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Slot, K.A.; Voorendt, M.; de Boer-Brouwer, M.; van Vugt, H.H.; Teerds, K.J. Estrous cycle dependent changes in expression and distribution of Fas, Fas ligand, Bcl-2, Bax, and pro- and active caspase-3 in the rat ovary. J. Endocrinol. 2006, 188, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Van Nassauw, L.; Tao, L.; Harrisson, F. Distribution of apoptosis-related proteins in the quail ovary during folliculogenesis: Bcl-2, Bax and CPP32. Acta Histochem. 1999, 101, 103–112. [Google Scholar] [CrossRef]
- Gomez, B.P.; Riggins, R.B.; Shajahan, A.N.; Klimach, U.; Wang, A.; Crawford, A.C.; Zhu, Y.; Zwart, A.; Wang, M.; Clarke, R. Human X-box binding protein-1 confers both estrogen independence and antiestrogen resistance in breast cancer cell lines. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 4013–4027. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cheng, D.; Zhou, S.; Zhu, B.; Hu, T.; Yang, Q. Overexpression of X-box binding protein 1 (XBP1) correlates to poor prognosis and up-regulation of PI3K/mTOR in human osteosarcoma. Int. J. Mol. Sci. 2015, 16, 28635–28646. [Google Scholar] [CrossRef] [PubMed]
- Heber-Katz, E.; Zhang, Y.; Bedelbaeva, K.; Song, F.; Chen, X.; Stocum, D.L. Cell cycle regulation and regeneration. Curr. Top. Microbiol. Immunol. 2013, 367, 253–276. [Google Scholar] [PubMed]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhang, L.; Ren, P.; Zhong, T.; Li, Z.; Wang, Z.; Li, J.; Liu, X.; Zhao, K.; Zhang, W.; et al. Enterovirus 71 mediates cell cycle arrest in S phase through non-structural protein 3D. Cell Cycle 2015, 14, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, H.; Zhang, Q.; Huang, Y.; Dong, J.; Liang, Y.; Liu, H.J.; Tong, D. Porcine epidemic diarrhea virus N protein prolongs S phase cell cycle, induces endoplasmic reticulum stress, and up-regulates interleukin-8 expression. Vet. Microbiol. 2013, 164, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.H.; Wang, L.; Riaz, H.; Wu, J.B.; Yuan, Y.F.; Han, L.; Wang, Y.L.; Zhao, Y.; Dan, Y.; Huo, L.J. Knockdown of CEBPβ by RNAi in porcine granulosa cells resulted in S phase cell cycle arrest and decreased progesterone and estradiol synthesis. J. Steroid Biochem. Mol. Biol. 2014, 143, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.G.; Walker, C.L. Cyclins and cell cycle checkpoints. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 295–312. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.J.; Lennard, D.E.; Lee, C.A.; Tiano, H.F.; Morham, S.G.; Wetsel, W.C.; Langenbach, R. Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1β. Endocrinology 1999, 140, 2685–2695. [Google Scholar] [CrossRef] [PubMed]
- Sirois, J.; Dore, M. The late induction of prostaglandin G/H synthase-2 in equine preovulatory follicles supports its role as a determinant of the ovulatory process. Endocrinology 1997, 138, 4427–4434. [Google Scholar] [CrossRef] [PubMed]





| Group | Live Cells (%) | Apoptosis Cells (%) |
|---|---|---|
| shRNA-3 group | 76.9 ± 0.68% | 18.83 ± 0.38% * |
| shRNA-negative group | 83.4 ± 0.95% | 13.67 ± 0.77% |
| Title | G1 (%) | S (%) | G2 (%) |
|---|---|---|---|
| shRNA-3 group | 74.58 ± 0.61% * | 15.54 ± 0.7% * | 9.88 ± 0.15% |
| shRNA-negative group | 81. 33 ± 0.68% | 6.82 ± 0.85% | 11.85 ± 0.16% |
| Group | Target Sequence(5′–3′) |
|---|---|
| shRNA-1 | GGAAGAAGAGAACCACAAACT |
| shRNA-2 | GCCAAGCTGGAAGCCATTAAT |
| shRNA-3 | GGCATCTCAAACCTGCTTTCA |
| shRNA-negative | GATGAAATGGGTAAGTACA |
| Gene | GenBank Accession No. | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|---|
| β-actin | NM_007393 | GCAAGCAGGAGTACGATGAG | CCATGCCAATGTTGTCTCTT |
| XBP1 | NM_001271730 | GAGCAGCAAGTGGTGGATTT | AAAGGGAGGCTGGTAAGGAA |
| Star | NM_011485.4 | CTTGGCTGCTCAGTATTGAC | TGGTGGACAGTCCTTAACAC |
| Cyp19a1 | NM_007810.3 | GACACATCATGCTGGACACC | CAAGTCCTTGACGGATCGTT |
| Cyp11a1 | NM_019779.3 | CGATACTCTTCTCATGCGAG | CTTTCTTCCAGGCATCTGAAC |
| Cyclin A1 | Z26580.1 | GCCTTCACCATTCATGTGGAT | TTGCTGCGGGTAAAGAGACAG |
| Cyclin B1 | NM_172301.3 | AAGGTGCCTGTGTGTGAACC | GTCAGCCCCATCATCTGCG |
| Cyclin D2 | NM_009829.3 | ACACCGACAACTCTGTGAAGC | GCCAGGTTCCACTTCAGCTTA |
| Cyclin E | NM_007633 | GTGGCTCCGACCTTTCAGTC | CACAGTCTTGTCAATCTTGGCA |
| Caspase-3 | NM_001284409.1 | TGACTGGAAAGCCGAAACTC | GCAAGCCATCTCCTCATCAG |
| Bcl-2 | NM_009741.4 | CGAGAAGAAGAGGGAATCACAGG | AATCCGTAGGAATCCCAACC |
| Has2 | NM_008216.3 | ACCCTGCCTCATCTGTGGAGA | TGTTGGTAAGGTGCCTGTCGT |
| Ptgs2 | NM_011198.3 | CTCTATCACTGGCACCCCCTG | GAAGCGTTTGCGGTACTCATT |
| CHOP | NM_007837.3 | AGCTGGAAGCCTGGTATGAGGA | AGCTAGGGACGCAGGGTCAA |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Zhao, F.; Lin, P.; Zhang, G.; Tang, K.; Wang, A.; Jin, Y. Knockdown of XBP1 by RNAi in Mouse Granulosa Cells Promotes Apoptosis, Inhibits Cell Cycle, and Decreases Estradiol Synthesis. Int. J. Mol. Sci. 2017, 18, 1152. https://doi.org/10.3390/ijms18061152
Wang N, Zhao F, Lin P, Zhang G, Tang K, Wang A, Jin Y. Knockdown of XBP1 by RNAi in Mouse Granulosa Cells Promotes Apoptosis, Inhibits Cell Cycle, and Decreases Estradiol Synthesis. International Journal of Molecular Sciences. 2017; 18(6):1152. https://doi.org/10.3390/ijms18061152
Chicago/Turabian StyleWang, Nan, Fan Zhao, Pengfei Lin, Guangle Zhang, Keqiong Tang, Aihua Wang, and Yaping Jin. 2017. "Knockdown of XBP1 by RNAi in Mouse Granulosa Cells Promotes Apoptosis, Inhibits Cell Cycle, and Decreases Estradiol Synthesis" International Journal of Molecular Sciences 18, no. 6: 1152. https://doi.org/10.3390/ijms18061152
APA StyleWang, N., Zhao, F., Lin, P., Zhang, G., Tang, K., Wang, A., & Jin, Y. (2017). Knockdown of XBP1 by RNAi in Mouse Granulosa Cells Promotes Apoptosis, Inhibits Cell Cycle, and Decreases Estradiol Synthesis. International Journal of Molecular Sciences, 18(6), 1152. https://doi.org/10.3390/ijms18061152