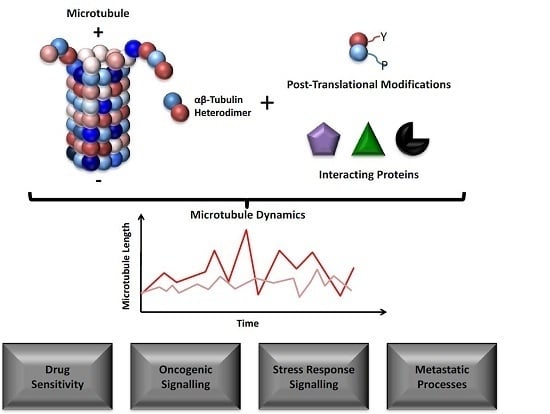
An Emerging Role for Tubulin Isotypes in Modulating Cancer Biology and Chemotherapy Resistance
Abstract
:1. Introduction
2. Tubulin Isotype Expression in Cancer
2.1. Tubulin Isotypes and Drug Resistance
2.2. Microtubule Dynamics and Chemotherapy Resistance
2.3. Tubulin Isotypes in Tumour Biology
3. Tubulin and Oncogenic Signalling
4. Tubulin and Microenvironmental Stress Response
4.1. Tubulin and Hypoxia
4.2. Oxidative Stress and Microtubules
4.3. Tubulin Isotypes and Metabolism
4.4. Tubulin Isotypes and Mitochondrial Function
4.5. Tubulin Isotypes and Endoplasmic Reticulum Stress
4.6. Tubulin Isotypes and Autophagy
5. Tubulin and Metastasis
6. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| TBA | Tubulin binding agents |
| MAP | Microtubule associated protein |
References
- Nogales, E. Structural insights into microtubule function. Annu. Rev. Biochem. 2000, 69, 277–302. [Google Scholar] [CrossRef] [PubMed]
- Bowne-Anderson, H.; Hibbel, A.; Howard, J. Regulation of microtubule growth and catastrophe: Unifying theory and experiment. Trends Cell Biol. 2015, 25, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Downing, K.H.; Nogales, E. Tubulin structure: Insights into microtubule properties and functions. Curr. Opin. Struct. Biol. 1998, 8, 785–791. [Google Scholar] [CrossRef]
- Janke, C. The tubulin code: Molecular components, readout mechanisms, and functions. J. Cell Biol. 2014, 206, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Valiron, O.; Caudron, N.; Job, D. Microtubule dynamics. Cell. Mol. Life Sci. 2001, 58, 2069–2084. [Google Scholar] [CrossRef] [PubMed]
- Akhmanova, A.; Steinmetz, M.O. Control of microtubule organization and dynamics: Two ends in the limelight. Nat. Rev. Mol. Cell. Biol. 2015, 16, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Zich, J.; Hardwick, K.G. Getting down to the phosphorylated “nuts and bolts” of spindle checkpoint signalling. Trends Biochem. Sci. 2010, 35, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Gigant, B.; Wang, C.G.; Ravelli, R.B.G.; Roussi, F.; Steinmetz, M.O.; Curmi, P.A.; Sobel, A.; Knossow, M. Structural basis for the regulation of tubulin by vinblastine. Nature 2005, 435, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Luduena, R.F. A hypothesis on the origin and evolution of tubulin. Int. Rev. Cell Mol. Biol. 2013, 302, 41–185. [Google Scholar] [PubMed]
- Luduena, R.F. Are tubulin isotypes functionally significant. Mol. Biol. Cell 1993, 4, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Verdier-Pinard, P.; Pasquier, E.; Xiao, H.; Burd, B.; Villard, C.; Lafitte, D.; Miller, L.M.; Angeletti, R.H.; Horwitz, S.B.; Braguer, D. Tubulin proteomics: Towards breaking the code. Anal. Biochem. 2009, 384, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.F.; Cleveland, D.W. Identification of conserved isotype-defining variable region sequences for 4 vertebrate β-tubulin polypeptide classes. Proc. Natl. Acad. Sci. USA 1986, 83, 4327–4331. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.A.; Tian, G.L.; Cowan, N.J. The α- and β-tubulin folding pathways. Trends Cell Biol. 1997, 7, 479–484. [Google Scholar] [CrossRef]
- Serna, M.; Carranza, G.; Martin-Benito, J.; Janowski, R.; Canals, A.; Coll, M.; Zabala, J.C.; Valpuesta, J.M. The structure of the complex between α-tubulin, TBCE and TBCB reveals a tubulin dimer dissociation mechanism. J. Cell Sci. 2015, 128, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Vilmar, A.; Garcia-Foncillas, J.; Huarriz, M.; Santoni-Rugiu, E.; Sorensen, J.B. RT-PCR versus immunohistochemistry for correlation and quantification of ERCC1, BRCA1, TUBB3 and RRM1 in NSCLC. Lung Cancer 2012, 75, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, S.; Miyoshi, Y.; Egawa, C.; Ishitobi, M.; Taguchi, T.; Tamaki, Y.; Monden, M.; Noguchi, S. Prediction of response to docetaxel by quantitative analysis of class I and III β-tubulin isotype mRNA expression in human breast cancers. Clin. Cancer Res. 2003, 9, 2992–2997. [Google Scholar] [PubMed]
- Roque, D.M.; Bellone, S.; English, D.P.; Buza, N.; Cocco, E.; Gasparrini, S.; Bortolomai, I.; Ratner, E.; Silasi, D.A.; Azodi, M.; et al. Tubulin-β-III overexpression by uterine serous carcinomas is a marker for poor overall survival after platinum/taxane chemotherapy and sensitivity to epothilones. Cancer 2013, 119, 2582–2592. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, Y.; Oda, Y.; Basaki, Y.; Kobayashi, H.; Wake, N.; Kuwano, M.; Tsuneyoshi, M. Expression of β-tubulin isotypes in human primary ovarian carcinoma. Gynecol. Oncol. 2007, 105, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Izutsu, N.; Maesawa, C.; Shibazaki, M.; Oikawa, H.; Shoji, T.; Sugiyama, T.; Masuda, T. Epigenetic modification is involved in aberrant expression of class III β-tubulin, TUBB3, in ovarian cancer cells. Int. J. Oncol. 2008, 32, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, B.; Fang, Y.; Liu, Y.; Wang, Y.; Li, J.; Zhou, W.; Wang, X. Overexpression of class III β-tubulin, sox2, and nuclear survivin is predictive of taxane resistance in patients with stage III ovarian epithelial cancer. BMC Cancer 2015, 15, 536. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Marty, C.; Treilleux, I.; Dumontet, C.; Cardoso, F.; Fellous, A.; Gancberg, D.; Bissery, M.C.; Paesmans, M.; Larsimont, D.; Piccart, M.J.; et al. Microtubule-associated parameters as predictive markers of docetaxel activity in advanced breast cancer patients: Results of a pilot study. Clin. Breast Cancer 2002, 3, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Seve, P.; Reiman, T.; Lai, R.; Hanson, J.; Santos, C.; Johnson, L.; Dabbagh, L.; Sawyer, M.; Dumontet, C.; Mackey, J.R. Class III β-tubulin is a marker of paclitaxel resistance in carcinomas of unknown primary site. Cancer Chemother. Pharmacol. 2007, 60, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Levallet, G.; Bergot, E.; Antoine, M.; Creveuil, C.; Santos, A.O.; Beau-Faller, M.; de Fraipont, F.; Brambilla, E.; Levallet, J.; Morin, F.; et al. High TUBB3 expression, an independent prognostic marker in patients with early non-small cell lung cancer treated by preoperative chemotherapy, is regulated by K-RAS signaling pathway. Mol. Cancer Ther. 2012, 11, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Seve, P.; Isaac, S.; Tredan, O.; Souquet, P.J.; Pacheco, Y.; Perol, M.; Lafanechere, L.; Penet, A.; Peiller, E.L.; Dumontet, C. Expression of class IIIβ-tubulin is predictive of patient outcome in patients with non-small cell lung cancer receiving vinorelbine-based chemotherapy. Clin. Cancer Res. 2005, 11, 5481–5486. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.E.; Hong, J.Y.; Kim, K.; Kim, S.H.; Choi, W.Y.; Kim, M.J.; Jung, S.H.; Shim, H.J.; Bae, W.K.; Hwang, E.C.; et al. Class III β-tubulin is a predictive marker for taxane-based chemotherapy in recurrent and metastatic gastric cancer. BMC Cancer 2013, 13, 431. [Google Scholar] [CrossRef] [PubMed]
- Christoph, D.C.; Kasper, S.; Gauler, T.C.; Loesch, C.; Engelhard, M.; Theegarten, D.; Poettgen, C.; Hepp, R.; Peglow, A.; Loewendick, H.; et al. βV-tubulin expression is associated with outcome following taxane-based chemotherapy in non-small cell lung cancer. Br. J. Cancer 2012, 107, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Roque, D.M.; Buza, N.; Glasgow, M.; Bellone, S.; Bortolomai, I.; Gasparrini, S.; Cocco, E.; Ratner, E.; Silasi, D.A.; Azodi, M.; et al. Class III β-tubulin overexpression within the tumor microenvironment is a prognostic biomarker for poor overall survival in ovarian cancer patients treated with neoadjuvant carboplatin/paclitaxel. Clin. Exp. Metastasis 2014, 31, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Kavallaris, M.; Kuo, D.Y.S.; Burkhart, C.A.; Regl, D.L.; Norris, M.D.; Haber, M.; Horwitz, S.B. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific β-tubulin isotypes. J. Clin. Investig. 1997, 100, 1282–1293. [Google Scholar] [CrossRef] [PubMed]
- Mozzetti, S.; Ferlini, C.; Concolino, P.; Filippetti, F.; Raspaglio, G.; Prislei, S.; Gallo, D.; Martinelli, E.; Ranelletti, F.O.; Ferrandina, G.; et al. Class III β-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin. Cancer Res. 2005, 11, 298–305. [Google Scholar] [PubMed]
- Ferrandina, G.; Zannoni, G.F.; Martinelli, E.; Paglia, A.; Gallotta, V.; Mozzetti, S.; Scambia, G.; Ferlini, C. Class III β-tubulin overexpression is a marker of poor clinical outcome in advanced ovarian cancer patients. Clin. Cancer Res. 2006, 12, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- De Donato, M.; Mariani, M.; Petrella, L.; Martinelli, E.; Zannoni, G.F.; Vellone, V.; Ferrandina, G.; Shahabi, S.; Scambia, G.; Ferlini, C. Class III β-tubulin and the cytoskeletal gateway for drug resistance in ovarian cancer. J. Cell. Physiol. 2012, 227, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Smith, S.M.; Preti, M.; Schwartz, P.; Rutherford, T.J.; Menato, G.; Danese, S.; Ma, S.L.; Yu, H.; Katsaros, D. Stathmin and tubulin expression and survival of ovarian cancer patients receiving platinum treatment with and without paclitaxel. Cancer 2009, 115, 2453–2463. [Google Scholar] [CrossRef] [PubMed]
- Aoki, D.; Oda, Y.; Hattori, S.; Taguchi, K.; Ohishi, Y.; Basaki, Y.; Oie, S.; Suzuki, N.; Kono, S.; Tsuneyoshi, M.; et al. Overexpression of class IIIβ-tubulin predicts good response to taxane-based chemotherapy in ovarian clear cell adenocarcinoma. Clin. Cancer Res. 2009, 15, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Sale, S.; Sung, R.; Shen, P.D.; Yu, K.; Wang, Y.; Duran, G.E.; Kim, J.H.; Fojo, T.; Oefner, P.J.; Sikic, B.I. Conservation of the class I β-tubulin gene in human populations and lack of mutations in lung cancers and paclitaxel-resistant ovarian cancers. Mol. Cancer Ther. 2002, 1, 215–225. [Google Scholar] [PubMed]
- Wang, W.; Zhang, H.; Wang, X.; Patterson, J.; Winter, P.; Graham, K.; Ghosh, S.; Lee, J.C.; Katsetos, C.D.; Mackey, J.R.; et al. Novel mutations involving βI-, βIIA-, or βIVB-tubulin isotypes with functional resemblance to βIII-tubulin in breast cancer. Protoplasma 2016, 254, 1163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Ruan, L.; Zheng, L.M.; Whyte, D.; Tzeng, C.M.; Zhou, X.W. Association between class IIIβ-tubulin expression and response to paclitaxel/vinorebine-based chemotherapy for non-small cell lung cancer: A meta-analysis. Lung Cancer 2012, 77, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Sève, P.; Lai, R.; Ding, K.; Winton, T.; Butts, C.; Mackey, J.; Dumontet, C.; Dabbagh, L.; Aviel-Ronen, S.; Seymour, L.; et al. Class IIIβ-tubulin expression and benefit from adjuvant cisplatin/vinorelbine chemotherapy in operable non–small cell lung cancer: Analysis of NCIC JBR.10. Clin. Cancer Res. 2007, 13, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Reiman, T.; Lai, R.; Veillard, A.S.; Paris, E.; Soria, J.C.; Rosell, R.; Taron, M.; Graziano, S.; Kratzke, R.; Seymour, L.; et al. Cross-validation study of class IIIβ-tubulin as a predictive marker for benefit from adjuvant chemotherapy in resected non-small-cell lung cancer: Analysis of four randomized trials. Ann. Oncol. 2012, 23, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarelli, V.; Hiser, L.; Smith, H.; Frankfurter, A.; Spano, A.; Correia, J.J.; Lobert, S. β-tubulin isotype classes II and V expression patterns in nonsmall cell lung carcinomas. Cell Motil. Cytoskelet. 2008, 65, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Sasada, T.; Kawahara, A.; Takamori, S.; Hattori, S.; Ikeda, J.; Itoh, K.; Yamada, A.; Kage, M.; Kuwano, M.; et al. Expression of ERCC1 and ulin in non-small cell lung cancer patients treated with carboplatin and paclitaxel. Lung Cancer 2009, 64, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Luo, X.P.; Xian, L. The prognostic role of the class IIIβ-tubulin in non-small cell lung cancer (NSCLC) patients receiving the taxane/vinorebine-based chemotherapy: A meta-analysis. PLoS ONE 2014, 9, e93997. [Google Scholar]
- Guo, N.; Zhang, W.; Zhang, B.; Li, Y.; Tang, J.; Li, S.; Zhao, Y.; Zhao, Y.; Xia, H.; Yu, C. EGFR and K-RAS mutations and ERCC1, TUBB3, TYMS, RRM1 and EGFR mRNA expression in non-small cell lung cancer: Correlation with clinical response to gefitinib or chemotherapy. Mol. Clin. Oncol. 2015, 3, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, T.; Yoshimasu, T.; Oura, S.; Kokawa, Y.; Kawago, M.; Hirai, Y.; Miyasaka, M.; Aoishi, Y.; Kiyoi, M.; Nishiguchi, H.; et al. Class IIIβ-tubulin expression in non-small cell lung cancer: A predictive factor for paclitaxel response. Anticancer Res. 2015, 35, 2669–2674. [Google Scholar]
- Jiang, H.; Yu, X.M.; Zhou, X.M.; Wang, X.H.; Su, D. Correlation between microtubule-associated gene expression and chemosensitivity of patients with stage II non-small cell lung cancer. Exp. Ther. Med. 2013, 5, 1506–1510. [Google Scholar] [PubMed]
- Paradiso, A.; Mangia, A.; Chiriatti, A.; Tommasi, S.; Zito, A.; Latorre, A.; Schittulli, F.; Lorusso, V. Biomarkers predictive for clinical efficacy of taxol-based chemotherapy in advanced breast cancer. Ann. Oncol. 2005, 16, 14–19. [Google Scholar] [CrossRef]
- He, W.; Zhang, D.; Jiang, J.; Liu, P.; Wu, C. The relationships between the chemosensitivity of human gastric cancer to paclitaxel and the expressions of class III β-tubulin, MAPT, and survivin. Med. Oncol. 2014, 31, 950. [Google Scholar] [CrossRef]
- Tsourlakis, M.C.; Weigand, P.; Grupp, K.; Kluth, M.; Steurer, S.; Schlomm, T.; Graefen, M.; Huland, H.; Salomon, G.; Steuber, T.; et al. βIII-tubulin overexpression is an independent predictor of prostate cancer progression tightly linked to ERG fusion status and PTEN deletion. Am. J. Pathol. 2014, 184, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Gan, P.P.; McCarroll, J.A.; Byrne, F.L.; Garner, J.; Kavallaris, M. Specific β-tubulin isotypes can functionally enhance or diminish epothilone b sensitivity in non-small cell lung cancer cells. PLoS ONE 2011, 6, e21717. [Google Scholar] [CrossRef] [PubMed]
- Gan, P.P.; Kavallaris, M. Tubulin-targeted drug action: Functional significance of class II and class IVb β-tubulin in vinca alkaloid sensitivity. Cancer Res. 2008, 68, 9817–9824. [Google Scholar] [CrossRef] [PubMed]
- Don, S.; Verrills, N.M.; Liaw, T.Y.E.; Liu, M.L.M.; Norris, M.D.; Haber, M.; Kavallaris, M. Neuronal-associated microtubule proteins class IIIβ-tubulin and MAP2c in neuroblastoma: Role in resistance to microtubule-targeted drugs. Mol. Cancer Ther. 2004, 3, 1137–1146. [Google Scholar] [PubMed]
- Bhattacharya, R.; Cabral, F. A ubiquitous β-tubulin disrupts microtubule assembly and inhibits cell proliferation. Mol. Biol. Cell 2004, 15, 3123–3131. [Google Scholar] [CrossRef] [PubMed]
- Lobert, S.; Jefferson, B.; Morris, K. Regulation of β-tubulin isotypes by micro-RNA 100 in MCF7 breast cancer cells. Cytoskeleton 2011, 68, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Verrills, N.M.; Walsh, B.J.; Cobon, G.S.; Hains, P.G.; Kavallaris, M. Proteome analysis of vinca alkaloid response and resistance in acute lymphoblastic leukemia reveals novel cytoskeletal alterations. J. Biol. Chem. 2003, 278, 45082–45093. [Google Scholar] [CrossRef] [PubMed]
- Mozzetti, S.; Iantomasi, R.; de Maria, I.; Prislei, S.; Mariani, M.; Camperchioli, A.; Bartollino, S.; Gallo, D.; Scambia, G.; Ferlini, C. Molecular mechanisms of patupilone resistance. Cancer Res. 2008, 68, 10197–10204. [Google Scholar] [CrossRef] [PubMed]
- Kanakkanthara, A.; Northcote, P.T.; Miller, J.H. βII-tubulin and βIII-tubulin mediate sensitivity to peloruside A and laulimalide, but not paclitaxel or vinblastine, in human ovarian carcinoma cells. Mol. Cancer Ther. 2012, 11, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xie, J.; Pan, Q.; Wang, B.; Hu, D.; Hu, X. Anticancer agent shikonin is an incompetent inducer of cancer drug resistance. PLoS ONE 2013, 8, e52706. [Google Scholar] [CrossRef] [PubMed]
- Galmarini, C.M.; Kamath, K.; Vanier-Viornery, A.; Hervieu, V.; Peiller, E.; Falette, N.; Puisieux, A.; Jordan, M.A.; Dumontet, C. Drug resistance associated with loss of p53 involves extensive alterations in microtubule composition and dynamics. Br. J. Cancer 2003, 88, 1793–1799. [Google Scholar] [CrossRef] [PubMed]
- Haber, M.; Burkhart, C.A.; Regl, D.L.; Madafiglio, J.; Norris, M.D.; Horwitz, S.B. Altered expression of Mβ2, the class II β-tubulin isotype, in a murine J774.2 cell line with a high level of taxol resistance. J. Biol. Chem. 1995, 270, 31269–31275. [Google Scholar] [CrossRef] [PubMed]
- Sharbeen, G.; McCarroll, J.; Liu, J.; Youkhana, J.; Limbri, L.F.; Biankin, A.V.; Johns, A.; Kavallaris, M.; Goldstein, D.; Phillips, P.A. Delineating the role of βIV-tubulins in pancreatic cancer: βIVb-tubulin inhibition sensitizes pancreatic cancer cells to vinca alkaloids. Neoplasia 2016, 18, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Narvi, E.; Jaakkola, K.; Winsel, S.; Oetken-Lindholm, C.; Halonen, P.; Kallio, L.; Kallio, M.J. Altered TUBB3 expression contributes to the epothilone response of mitotic cells. Br. J. Cancer 2013, 108, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Kavallaris, M.; Burkhart, C.A.; Horwitz, S.B. Antisense oligonucleotides to class III β-tubulin sensitize drug-resistant cells to taxol. Br. J. Cancer 1999, 80, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- McCarroll, J.A.; Gan, P.P.; Liu, M.; Kavallaris, M. β III-tubulin is a multifunctional protein involved in drug sensitivity and tumorigenesis in non-small cell lung cancer. Cancer Res. 2010, 70, 4995–5003. [Google Scholar] [CrossRef] [PubMed]
- Mhaidat, N.M.; Thorne, R.F.; de Bock, C.E.; Zhang, X.D.; Hersey, P. Melanoma cell sensitivity to docetaxel-induced apoptosis is determined by class III β-tubulin levels. FEBS Lett. 2008, 582, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, K.; Maesawa, C.; Shibazaki, M.; Maeda, F.; Takahashi, K.; Akasaka, T.; Masuda, T. Loss of class III β-tubulin induced by histone deacetylation is associated with chemosensitivity to paclitaxel in malignant melanoma cells. J. Investig. Dermatol. 2009, 129, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- McCarroll, J.A.; Sharbeen, G.; Liu, J.; Youkhana, J.; Goldstein, D.; McCarthy, N.; Limbri, L.F.; Dischl, D.; Ceyhan, G.O.; Erkan, M.; et al. β III-tubulin: A novel mediator of chemoresistance and metastases in pancreatic cancer. Oncotarget 2015, 6, 2235–2249. [Google Scholar] [CrossRef] [PubMed]
- Hari, M.; Yang, H.; Zeng, C.; Canizales, M.; Cabral, F. Expression of class III β-tubulin reduces microtubule assembly and confers resistance to paclitaxel. Cell Motil. Cytoskelet. 2003, 56, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Blade, K.; Menick, D.R.; Cabral, F. Overexpression of class I, II or IVb β-tubulin isotypes in CHO cells is insufficient to confer resistance to paclitaxel. J. Cell Sci. 1999, 112, 2213–2221. [Google Scholar] [PubMed]
- Ranganathan, S.; McCauley, R.A.; Dexter, D.W.; Hudes, G.R. Modulation of endogenous β-tubulin isotype expression as a result of human βIIIcDNA transfection into prostate carcinoma cells. Br. J. Cancer 2001, 85, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Gay, D.A.; Sisodia, S.S.; Cleveland, D.W. Autoregulatory control of β-tubulin mRNA stability is linked to translation elongation. Proc. Natl. Acad. Sci. USA 1989, 86, 5763–5767. [Google Scholar] [CrossRef] [PubMed]
- Lobert, S.; Graichen, M.E.; Morris, K. Coordinated regulation of β-tubulin isotypes and epithelial-to-mesenchymal transition protein zeb1 in breast cancer cells. Biochemistry 2013, 52, 5482–5490. [Google Scholar] [CrossRef] [PubMed]
- Seve, P.; Mackey, J.; Isaac, S.; Tredan, O.; Souquet, P.J.; Perol, M.; Lai, R.; Voloch, A.; Dumontet, C. Class III β-tubulin expression in tumor cells predicts response and outcome in patients with non-small cell lung cancer receiving paclitaxel. Mol. Cancer Ther. 2005, 4, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.; Miller, H.P.; Banerjee, A.; Luduena, R.F.; Wilson, L. Microtubule dynamics in vitro are regulated by the tubulin isotype composition. Proc. Natl. Acad. Sci. USA 1994, 91, 11358–11362. [Google Scholar] [CrossRef] [PubMed]
- Derry, W.B.; Wilson, L.; Khan, I.A.; Luduena, R.F.; Jordan, M.A. Taxol differentially modulates the dynamics of microtubules assembled from unfractionated and purified β-tubulin isotypes. Biochemistry 1997, 36, 3554–3562. [Google Scholar] [CrossRef] [PubMed]
- Falconer, M.M.; Echeverri, C.J.; Brown, D.L. Differential sorting of β tubulin isotypes into colchicine-stable microtubules during neuronal and muscle differentiation of embryonal carcinoma-cells. Cell Motil. Cytoskelet. 1992, 21, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Rezania, V.; Azarenko, O.; Jordan, M.A.; Bolterauer, H.; Luduena, R.F.; Huzil, J.T.; Tuszynski, J.A. Microtubule assembly of isotypically purified tubulin and its mixtures. Biophys. J. 2008, 95, 1993–2008. [Google Scholar] [CrossRef] [PubMed]
- Freedman, H.; Huzil, J.T.; Luchko, T.; Luduena, R.F.; Tuszynski, J.A. Identification and characterization of an intermediate taxol binding site within microtubule nanopores and a mechanism for tubulin isotype binding selectivity. J. Chem. Inf. Model. 2009, 49, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Pamula, M.C.; Ti, S.C.; Kapoor, T.M. The structured core of human β tubulin confers isotype-specific polymerization properties. J. Cell Biol. 2016, 213, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Roach, M.C.; Trcka, P.; Luduena, R.F. Increased microtubule assembly in bovine brain tubulin lacking the type-III isotype of β-tubulin. J. Biol. Chem. 1990, 265, 1794–1799. [Google Scholar] [PubMed]
- Lu, Q.; Luduena, R.F. Removal of βIII isotype enhances taxol-induced microtubule assembly. Cell Struct. Funct. 1993, 18, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Luduena, R.F. Kinetics of colchicine binding to purified β-tubulin isotypes from bovine brain. J. Biol. Chem. 1992, 267, 13335–13339. [Google Scholar] [PubMed]
- Gan, P.P.; McCarroll, J.A.; Po’uha, S.T.; Kamath, K.; Jordan, M.A.; Kavallaris, M. Microtubule dynamics, mitotic arrest, and apoptosis: Drug-induced differential effects of β III-tubulin. Mol. Cancer Ther. 2010, 9, 1339–1348. [Google Scholar] [CrossRef] [PubMed]
- Bouchet, B.P.; Puisieux, A.; Galmarini, C.M. β III-tubulin is required for interphase microtubule dynamics in untransformed human mammary epithelial cells. Eur. J. Cell Biol. 2011, 90, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Ludueña, R.F. Different effects of vinblastine on the polymerization of isotypically purified tubulins from bovine brain. Investig. New Drugs 2003, 21, 3–13. [Google Scholar] [CrossRef]
- Wilson, L.; Lopus, M.; Miller, H.P.; Azarenko, O.; Riffle, S.; Smith, J.A.; Jordan, M.A. Effects of eribulin on microtubule binding and dynamic instability are strengthened in the absence of the β III tubulin isotype. Biochemistry 2015, 54, 6482–6489. [Google Scholar] [CrossRef] [PubMed]
- Lopus, M.; Smiyun, G.; Miller, H.; Oroudjev, E.; Wilson, L.; Jordan, M.A. Mechanism of action of ixabepilone and its interactions with the βIII-tubulin isotype. Cancer Chemother. Pharmacol. 2015, 76, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Kamath, K.; Wilson, L.; Cabral, F.; Jordan, M.A. β III-tubulin induces paclitaxel resistance in association with reduced effects on microtubule dynamic instability. J. Biol. Chem. 2005, 280, 12902–12907. [Google Scholar] [CrossRef] [PubMed]
- Stengel, C.; Newman, S.P.; Leese, M.P.; Potter, B.V.L.; Reed, M.J.; Purohit, A. Class III β-tubulin expression and in vitro resistance to microtubule targeting agents. Br. J. Cancer 2010, 102, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Dhoore, A.; Engelborghs, Y. Interaction of desacetamidocolchicine, a fast binding analog of colchicine with isotypically pure tubulin dimers αβII, αβIII and αβIV. J. Biol. Chem. 1994, 269, 10324–10329. [Google Scholar] [PubMed]
- Banerjee, A.; Engelborghs, Y.; Dhoore, A.; Fitzgerald, T.J. Interactions of a bicyclic analog of colchicine with β-tubulin isoforms αβII, αβIII and αβIV. Eur. J. Biochem. 1997, 246, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Schwarz, P.M.; Luduena, R.F. Interaction of nocodazole with tubulin isotypes. Drug Dev. Res. 2002, 55, 91–96. [Google Scholar] [CrossRef]
- Laing, N.; Dahllöf, B.; Hartley-Asp, B.; Ranganathan, S.; Tew, K.D. Interaction of estramustine with tubulin isotypes. Biochemistry 1997, 36, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Tulub, A.A.; Stefanov, V.E. Cisplatin stops tubulin assembly into microtubules. A new insight into the mechanism of antitumor activity of platinum complexes. Int. J. Biol. Macromol. 2001, 28, 191–198. [Google Scholar] [CrossRef]
- Boekelheide, K.; Arcila, M.E.; Eveleth, J. CIS-diamminedichloroplatinum (II) (cisplatin) alters microtubule assembly dynamics. Toxicol. Appl. Pharmacol. 1992, 116, 146–151. [Google Scholar] [CrossRef]
- Gan, P.P.; Pasquier, E.; Kavallaris, M. Class III β-tubulin mediates sensitivity to chemotherapeutic drugs in non-small cell lung cancer. Cancer Res. 2007, 67, 9356–9363. [Google Scholar] [CrossRef] [PubMed]
- Yeh, L.-C.C.; Banerjee, A.; Prasad, V.; Tuszynski, J.A.; Weis, A.L.; Bakos, T.; Yeh, I.T.; Ludueña, R.F.; Lee, J.C. Effect of CH-35, a novel anti-tumor colchicine analogue, on breast cancer cells overexpressing the βIII isotype of tubulin. Investig. New Drugs 2016, 34, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Kumbhar, B.V.; Borogaon, A.; Panda, D.; Kunwar, A. Exploring the origin of differential binding affinities of human tubulin isotypes αβII, αβIII and αβIV for DAMA-colchicine using homology modelling, molecular docking and molecular dynamics simulations. PLoS ONE 2016, 11, e0156048. [Google Scholar] [CrossRef] [PubMed]
- Das, L.; Bhattacharya, B.; Basu, G. Rationalization of paclitaxel insensitivity of yeast β-tubulin and human βIII-tubulin isotype using principal component analysis. BMC Res. Notes 2012, 5, 395. [Google Scholar] [CrossRef] [PubMed]
- Geyer, E.A.; Burns, A.; Lalonde, B.A.; Ye, X.; Piedra, F.-A.; Huffaker, T.C.; Rice, L.M. A mutation uncouples the tubulin conformational and GTpase cycles, revealing allosteric control of microtubule dynamics. eLife 2015, 4, e10113. [Google Scholar] [CrossRef] [PubMed]
- Ti, S.C.; Pamula, M.C.; Howes, S.C.; Duellberg, C.; Cade, N.I.; Kleiner, R.E.; Forth, S.; Surrey, T.; Nogales, E.; Kapoor, T.M. Mutations in human tubulin proximal to the kinesin-binding site alter dynamic instability at microtubule plus- and minus-ends. Dev. Cell 2016, 37, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Mahapatra, P.; Manna, T.; Chakrabarti, P.; Bhattacharyya, L.; Banerjee, A.; Basu, G.; Roy, S. Conformational properties of -tubulin tail peptide: Implications for tail-body interaction. Biochemistry 2001, 40, 15512–15519. [Google Scholar] [CrossRef] [PubMed]
- Freedman, H.; Luchko, T.; Luduena, R.F.; Tuszynski, J.A. Molecular dynamics modeling of tubulin C-terminal tail interactions with the microtubule surface. Proteins Struct. Funct. Bioinf. 2011, 79, 2968–2982. [Google Scholar] [CrossRef] [PubMed]
- Luchko, T.; Huzil, J.T.; Stepanova, M.; Tuszynski, J. Conformational analysis of the carboxy-terminal tails of human β-tubulin isotypes. Biophys. J. 2008, 94, 1971–1982. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, B.; Sackett, D.L.; Wolff, J. Tubulin, hybrid dimers and tubulin S. J. Biol. Chem. 1985, 260, 10208–10216. [Google Scholar] [PubMed]
- Wolff, J.; Sackett, D.L.; Knipling, L. Cation selective promotion of tubulin polymerization by alkali metal chlorides. Protein Sci. 1996, 5, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Mejillano, M.R.; Himes, R.H. Assembly properties of tubulin after carboxyl group modification. J. Biol. Chem. 1991, 266, 657–664. [Google Scholar] [PubMed]
- Mejillano, M.R.; Tolo, E.T.; Williams, R.C.; Himes, R.H. The conversion of tubulin carboxyl groups to amides has a stabilizing effect on microtubules. Biochemistry 1992, 31, 3478–3483. [Google Scholar] [CrossRef] [PubMed]
- Szasz, J.; Yaffe, M.B.; Elzinga, M.; Blank, G.S.; Sternlicht, H. Microtubule assembly is dependent on a cluster of basic residues in α-tubulin. Biochemistry 1986, 25, 4572–4582. [Google Scholar] [CrossRef] [PubMed]
- Sherman, G.; Rosenberry, T.L.; Sternlicht, H. Identification of lysine residues essential for microtubule assembly—Demonstration of enhanced reactivity during reductive methylation. J. Biol. Chem. 1983, 258, 2148–2156. [Google Scholar] [PubMed]
- Serrano, L.; Delatorre, J.; Maccioni, R.B.; Avila, J. Involvement of the carboxyl-terminal domain of tubulin in the regulation of its assembly. Proc. Natl. Acad. Sci. USA 1984, 81, 5989–5993. [Google Scholar] [CrossRef] [PubMed]
- Job, D.; Pabion, M.; Margolis, R.L. Generation of microtubule stability subclasses by microtubule-associated proteins—Implications for the microtubule dynamic instability model. J. Cell Biol. 1985, 101, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Stromberg, E.; Wallin, M. Differences in the effect of Ca2+ on isolated microtubules from cod and cow brain. Cell Motil. Cytoskelet. 1994, 28, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, J.; Chernov, K.G.; Joshi, V.; Delga, S.; Toma, F.; Pastre, D.; Curmi, P.A.; Savarin, P. The C-terminus of tubulin, a versatile partner for cationic molecules binding of τ, polyamines, and calcium. J. Biol. Chem. 2011, 286, 3065–3078. [Google Scholar] [CrossRef] [PubMed]
- Littauer, U.Z.; Giveon, D.; Thierauf, M.; Ginzburg, I.; Ponstingl, H. Common and distinct tubulin binding-sites for microtubule-associated proteins. Proc. Natl. Acad. Sci. USA 1986, 83, 7162–7166. [Google Scholar] [CrossRef] [PubMed]
- Chau, M.F.; Radeke, M.J.; de Ines, C.; Barasoain, I.; Kohlstaedt, L.A.; Feinstein, S.C. The microtubule-associated protein τ cross-links to two distinct sites on each α and β tubulin monomer via separate domains. Biochemistry 1998, 37, 17692–17703. [Google Scholar] [CrossRef] [PubMed]
- Laurin, Y.; Eyer, J.; Robert, C.H.; Prevost, C.; Sacquin-Mora, S. Mobility and core-protein binding patterns of disordered C-terminal tails in β-tubulin isotypes. Biochemistry 2017, 56, 1746–1756. [Google Scholar] [CrossRef] [PubMed]
- Joe, P.A.; Banerjee, A.; Luduena, R.F. Roles of β-tubulin residues Ala428 and Thr429 in microtubule formation in vivo. J. Biol. Chem. 2009, 284, 4283–4291. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Bechstedt, S.; Chaaban, S.; Brouhard, G.J. Microtubule-associated proteins control the kinetics of microtubule nucleation. Nat. Cell Biol. 2015, 17, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Chanez, B.; Goncalves, A.; Badache, A.; Verdier-Pinard, P. Eribulin targets a ch-TOG-dependent directed migration of cancer cells. Oncotarget 2015, 6, 41667–41678. [Google Scholar] [PubMed]
- Lee, K.M.; Cao, D.; Itami, A.; Pour, P.M.; Hruban, R.H.; Maitra, A.; Ouellette, M.M. Class III β-tubulin, a marker of resistance to paclitaxel, is overexpressed in pancreatic ductal adenocarcinoma and intraepithelial neoplasia. Histopathology 2007, 51, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Kanojia, D.; Morshed, R.A.; Zhang, L.J.; Miska, J.M.; Qiao, J.; Kim, J.W.; Pytel, P.; Balyasnikova, I.V.; Lesniak, M.S.; Ahmed, A.U. β III-tubulin regulates breast cancer metastases to the brain. Mol. Cancer Ther. 2015, 14, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Tang, Y.; Chen, W.W.; Wang, Y.L.; Yang, L.; Li, X.; Song, G.L.; Kuang, J. TUBB3 regulation by the ERK and AKT signaling pathways: A mechanism involved in the effect of arginine ADP-ribosyltransferase 1 (Art1) on apoptosis of colon carcinoma CT26 cells. Tumour Biol. 2016, 37, 2353–2363. [Google Scholar] [CrossRef] [PubMed]
- McCarroll, J.A.; Gan, P.P.; Erlich, R.B.; Liu, M.; Dwarte, T.; Sagnella, S.S.; Akerfeldt, M.C.; Yang, L.; Parker, A.L.; Chang, M.H.; et al. TUBB3/β III-tubulin acts through the PTEN/AKT signaling axis to promote tumorigenesis and anoikis resistance in non-small cell lung cancer. Cancer Res. 2015, 75, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Y.; Chu, Z.G.; Han, J.; Dang, Y.M.; Yan, H.; Zhang, Q.; Liang, G.P.; Huang, Y.S. The p38/MAPK pathway regulates microtubule polymerization through phosphorylation of MAP4 and OP18 in hypoxic cells. Cell. Mol. Life Sci. 2010, 67, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.D.; Xu, X.; Dang, Y.M.; Zhang, Y.M.; Zhang, J.P.; Hu, J.Y.; Zhang, Q.; Dai, X.; Teng, M.; Zhang, D.X.; et al. MAP4 mechanism that stabilizes mitochondrial permeability transition in hypoxia: Microtubule enhancement and dynlt1 interaction with VDAC1. PLoS ONE 2011, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.O.; Shin, S.; Mercurio, A.M. Hypoxia stimulates carcinoma invasion by stabilizing microtubules and promoting the RAB11 trafficking of the α6β4 integrin. Cancer Res. 2005, 65, 2761–2769. [Google Scholar] [CrossRef] [PubMed]
- Raspaglio, G.; Filippetti, F.; Prislei, S.; Penci, R.; de Maria, I.; Cicchillitti, L.; Mozzetti, S.; Scambia, G.; Ferlini, C. Hypoxia induces class III β-tubulin gene expression by HIF-1α binding to its 3’ flanking region. Gene 2008, 409, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Bordji, K.; Grandval, A.; Cuhna-Alves, L.; Lechapt-Zalcman, E.; Bernaudin, M. Hypoxia-inducible factor-2 (HIF-2), but not HIF-1, is essential for hypoxic induction of class III β-tubulin expression in human glioblastoma cells. FEBS J. 2014, 281, 5220–5236. [Google Scholar] [CrossRef] [PubMed]
- Raspaglio, G.; Petrillo, M.; Martinelli, E.; Puma, D.D.L.; Mariani, M.; de Donato, M.; Filippetti, F.; Mozzetti, S.; Prislei, S.; Zannoni, G.F.; et al. Sox9 and HIF-2α regulate TUBB3 gene expression and affect ovarian cancer aggressiveness. Gene 2014, 542, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Raspaglio, G.; De Maria, I.; Filippetti, F.; Martinelli, E.; Zannoni, G.F.; Prislei, S.; Ferrandina, G.; Shahabi, S.; Scambia, G.; Ferlini, C. Hur regulates β-tubulin isotype expression in ovarian cancer. Cancer Res. 2010, 70, 5891–5900. [Google Scholar] [CrossRef] [PubMed]
- Hinman, M.N.; Lou, H. Diverse molecular functions of Hu proteins. Cell. Mol. Life Sci. 2008, 65, 3168–3181. [Google Scholar] [CrossRef] [PubMed]
- Katsetos, C.D.; del Valle, L.; Geddes, J.F.; Assimakopoulou, M.; Legido, A.; Boyd, J.C.; Balin, B.; Parikh, N.A.; Maraziotis, T.; de Chadarevian, J.P.; et al. Aberrant localization of the neuronal class III β-tubulin in astrocytomas—A marker for anaplastic potential. Arch. Pathol. Lab. Med. 2001, 125, 613–624. [Google Scholar] [PubMed]
- Patel, V.P.; Chu, C.T. Decreased SIRT2 activity leads to altered microtubule dynamics in oxidatively-stressed neuronal cells: Implications for Parkinson’s disease. Exp. Neurol. 2014, 257, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Drum, B.M.L.; Yuan, C.; Li, L.; Liu, Q.H.; Wordeman, L.; Santana, L.F. Oxidative stress decreases microtubule growth and stability in ventricular myocytes. J. Mol. Cell. Cardiol. 2016, 93, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Joe, P.A.; Banerjee, A.; Luduena, R.F. The roles of Cys124 and Ser239 in the functional properties of human βIII tubulin. Cell Motil. Cytoskelet. 2008, 65, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, J.H.; Jeong, W.W.; Choi, D.H.; Cha, H.J.; Kim, D.H.; Kwon, J.K.; Park, S.E.; Park, J.H.; Cho, H.R.; et al. Reactive oxygen species-dependent endog release mediates cisplatin-induced caspase-independent apoptosis in human head and neck squamous carcinoma cells. Int. J. Cancer 2008, 122, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Selimovic, D.; Hassan, M.; Haikel, Y.; Hengge, U.R. Taxol-induced mitochondrial stress in melanoma cells is mediated by activation of c-jun n-terminal kinase (JNK) and p38 pathways via uncoupling protein 2. Cell. Signal. 2008, 20, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Cicchillitti, L.; Penci, R.; Di Michele, M.; Filippetti, F.; Rotilio, D.; Donati, M.B.; Scambia, G.; Ferlini, C. Proteomic characterization of cytoskeletal and mitochondrial class III β-tubulin. Mol. Cancer Ther. 2008, 7, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Y.; Walss-Bass, C.; Luduena, R.F. The β isotypes of tubulin in neuronal differentiation. Cytoskeleton 2010, 67, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Infante, A.S.; Stein, M.S.; Zhai, Y.; Borisy, G.G.; Gundersen, G.G. Detyrosinated (Glu) microtubules are stabilized by an ATP-sensitive plus-end cap. J. Cell Sci. 2000, 113, 3907–3919. [Google Scholar] [PubMed]
- Aon, M.A.; Cortassa, S. Coherent and robust modulation of a metabolic network by cytoskeletal organization and dynamics. Biophys. Chem. 2002, 97, 213–231. [Google Scholar] [CrossRef]
- Sheldon, K.L.; Maldonado, E.N.; Lemasters, J.J.; Rostovtseva, T.K.; Bezrukov, S.M. Phosphorylation of voltage-dependent anion channel by serine/threonine kinases governs its interaction with tubulin. PLoS ONE 2011, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Rostovtseva, T.K.; Sheldon, K.L.; Hassanzadeh, E.; Monge, C.; Saks, V.; Bezrukov, S.M.; Sackett, D.L. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc. Natl. Acad. Sci. USA 2008, 105, 18746–18751. [Google Scholar] [CrossRef] [PubMed]
- Carre, M.; Andre, N.; Carles, G.; Borghi, H.; Brichese, L.; Briand, C.; Braguer, D. Tubulin is an inherent component of mitochondrial membranes that interacts with the voltage-dependent anion channel. J. Biol. Chem. 2002, 277, 33664–33669. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, E.N.; Sheldon, K.L.; DeHart, D.N.; Patnaik, J.; Manevich, Y.; Townsend, D.M.; Bezrukov, S.M.; Rostovtseva, T.K.; Lemasters, J.J. Voltage-dependent anion channels modulate mitochondrial metabolism in cancer cells- regulation by free tubulin and erastin. J. Biol. Chem. 2013, 288, 11920–11929. [Google Scholar] [CrossRef] [PubMed]
- Gurnev, P.A.; Rostovtseva, T.K.; Bezrukov, S.M. Tubulin-blocked state of VDAC studied by polymer and ATP partitioning. FEBS Lett. 2011, 585, 2363–2366. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, K.L.; Gurnev, P.A.; Bezrukov, S.M.; Sackett, D.L. Tubulin tail sequences and post-translational modifications regulate closure of mitochondrial voltage-dependent anion channel (VDAC). J. Biol. Chem. 2015, 290, 26784–26789. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y.; Shimizu, S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 2007, 12, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Morselli, E.; Kepp, O.; Vitale, I.; Rigoni, A.; Vacchelli, E.; Michaud, M.; Zischka, H.; Castedo, M.; Kroemer, G. Mitochondrial gateways to cancer. Mol. Asp. Med. 2010, 31, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Vertessy, B.G.; Bankfalvi, D.; Kovacs, J.; Low, P.; Lehotzky, A.; Ovadi, J. Pyruvate kinase as a microtubule destabilizing factor in vitro. Biochem. Biophys. Res. Commun. 1999, 254, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Marmillot, P.; Keith, T.; Srivastava, D.K.; Knull, H.R. Effect of tubulin on the activity of the muscle isoenzyme of lactate-dehydrogenase. Arch. Biochem. Biophys. 1994, 315, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Durrieu, C.; Berniervalentin, F.; Rousset, B. Microtubules bind glyceraldehyde-3-phosphate dehydrogenase and modulate its enzyme-activity and quaternary structure. Arch. Biochem. Biophys. 1987, 252, 32–40. [Google Scholar] [CrossRef]
- Muronetz, V.I.; Wang, Z.X.; Keith, T.J.; Knull, H.R.; Srivastava, D.K. Binding constants and stoichiometries of glyceraldehyde-3-phosphate dehydrogenase-tubulin complexes. Arch. Biochem. Biophys. 1994, 313, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, E.J.; Kelly, C.; Artalejo, C.R. Glyceraldehyde-3-phosphate dehydrogenase interacts with Rab2 and plays an essential role in endoplasmic reticulum to Golgi transport exclusive of its glycolytic activity. J. Biol. Chem. 2004, 279, 54046–54052. [Google Scholar] [CrossRef] [PubMed]
- Cueille, N.; Blanc, C.T.; Riederer, I.M.; Riederer, B.M. Microtubule-associated protein 1B binds glyceraldehyde-3-phosphate dehydrogenase. J. Proteom. Res. 2007, 6, 2640–2647. [Google Scholar] [CrossRef] [PubMed]
- Zala, D.; Hinckelmann, M.V.; Yu, H.; da Cunha, M.M.L.; Liot, G.; Cordelieres, F.P.; Marco, S.; Saudou, F. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell 2013, 152, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Glaser, P.E.; Han, X.L.; Gross, R.W. Tubulin is the endogenous inhibitor of the glyceraldehyde 3-phosphate dehydrogenase isoform that catalyzes membrane fusion: Implications for the coordinated regulation of glycolysis and membrane fusion. Proc. Natl. Acad. Sci. USA 2002, 99, 14104–14109. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, E.J.; Azizi, F.; Artalejo, C.R. Rab2 utilizes glyceraldehyde-3-phosphate dehydrogenase and protein kinase c iota to associate with microtubules and to recruit dynein. J. Biol. Chem. 2009, 284, 5876–5884. [Google Scholar] [CrossRef] [PubMed]
- Orosz, F.; Santamaria, B.; Ovadi, J.; Aragon, J.J. Phosphofructokinase from dictyostelium discoideum is a potent inhibitor of tubulin polymerization. Biochemistry 1999, 38, 1857–1865. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, J.; Low, P.; Pacz, A.; Horvath, I.; Olah, J.; Ovadi, J. Phosphoenolpyruvate-dependent tubulin-pyruvate kinase interaction at different organizational levels. J. Biol. Chem. 2003, 278, 7126–7130. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.L.; Turner, N.; McCarroll, J.A.; Kavallaris, M. βIII-tubulin alters glucose metabolism and stress response signaling to promote cell survival and proliferation in glucose-starved non-small cell lung cancer cells. Carcinogenesis 2016, 37, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Mccarroll, J.; Kavallaris, M. Microtubules and their role in cellular stress in cancer. Front. Oncol. 2014, 4, 153. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Filliol, D.; Miehe, M.; Rendon, A. Interaction of brain mitochondria with microtubules reconstituted from brain tubulin and MAP2 or TAU. Cell Motil. Cytoskelet. 1993, 24, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Rovini, A.; Savry, A.; Braguer, D.; Carre, M. Microtubule-targeted agents: When mitochondria become essential to chemotherapy. Biochim. Biophys. Acta 2011, 1807, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Strohecker, A.M.; Guo, J.Y.; Karsli-Uzunbas, G.; Price, S.M.; Chen, G.J.; Mathew, R.; McMahon, M.; White, E. Autophagy sustains mitochondrial glutamine metabolism and growth of BrafV600E—Driven lung tumors. Cancer Discov. 2013, 3, 1272–1285. [Google Scholar] [CrossRef] [PubMed]
- Karsli-Uzunbas, G.; Guo, J.Y.; Price, S.; Teng, X.; Laddha, S.V.; Khor, S.; Kalaany, N.Y.; Jacks, T.; Chan, C.S.; Rabinowitz, J.D.; et al. Autophagy is required for glucose homeostasis and lung tumor maintenance. Cancer Discov. 2014, 4, 914–927. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Granillo, M.; Grichine, A.; Guzun, R.; Usson, Y.; Tepp, K.; Chekulayev, V.; Shevchuk, I.; Karu-Varikmaa, M.; Kuznetsov, A.V.; Grimm, M.; et al. Studies of the role of tubulin β II isotype in regulation of mitochondrial respiration in intracellular energetic units in cardiac cells. J. Mol. Cell. Cardiol. 2012, 52, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Guzun, R.; Karu-Varikmaa, M.; Gonzalez-Granillo, M.; Kuznetsov, A.V.; Michel, L.; Cottet-Rousselle, C.; Saaremae, M.; Kaambre, T.; Metsis, M.; Grimm, M.; et al. Mitochondria-cytoskeleton interaction: Distribution of β-tubulins in cardiomyocytes and HL-1 cells. Biochim. Biophys. Acta 2011, 1807, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Varikmaa, M.; Bagur, R.; Kaambre, T.; Grichine, A.; Timohhina, N.; Tepp, K.; Shevchuk, I.; Chekulayev, V.; Metsis, M.; Boucher, F.; et al. Role of mitochondria-cytoskeleton interactions in respiration regulation and mitochondrial organization in striated muscles. Biochim. Biophys. Acta 2014, 1837, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Anmann, T.; Varikmaa, M.; Timohhina, N.; Tepp, K.; Shevchuk, I.; Chekulayev, V.; Saks, V.; Kaambre, T. Formation of highly organized intracellular structure and energy metabolism in cardiac muscle cells during postnatal development of rat heart. Biochim. Biophys. Acta 2014, 1837, 1350–1361. [Google Scholar] [CrossRef] [PubMed]
- Sirajuddin, M.; Rice, L.M.; Vale, R.D. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat. Cell Biol. 2014, 16, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Feizabadi, M.S. The contribution of the C-terminal tails of microtubules in altering the force production specifications of multiple kinesin-1. Cell Biochem. Biophys. 2016, 74, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Pilling, A.D.; Horiuchi, D.; Lively, C.M.; Saxton, W.M. Kinesin-1 and dynein are the primary motors for fast transport of mitochondria in drosophila motor axons. Mol. Biol. Cell 2006, 17, 2057–2068. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kanai, Y.; Okada, Y.; Nonaka, S.; Takeda, S.; Harada, A.; Hirokawa, N. Targeted disruption of mouse conventional kinesin heavy chain, KIF5b, results in abnormal perinuclear clustering of mitochondria. Cell 1998, 93, 1147–1158. [Google Scholar] [CrossRef]
- Tischfield, M.A.; Baris, H.N.; Wu, C.; Rudolph, G.; van Maldergem, L.; He, W.; Chan, W.M.; Andrews, C.; Demer, J.L.; Robertson, R.L.; et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and axon guidance. Cell 2010, 140, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Tischfield, M.A.; Engle, E.C. Distinct α- and β-tubulin isotypes are required for the positioning, differentiation and survival of neurons: New support for the ‘multi-tubulin’ hypothesis. Biosci. Rep. 2010, 30, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Niwa, S.; Takahashi, H.; Hirokawa, N. β-Tubulin mutations that cause severe neuropathies disrupt axonal transport. EMBO J. 2013, 32, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.; Balasubramanian, R.; Chan, W.M.; Kang, P.B.; Andrews, C.; Webb, B.D.; MacKinnon, S.E.; Oystreck, D.T.; Rankin, J.; Crawford, T.O.; et al. A novel syndrome caused by the E410K amino acid substitution in the neuronal β-tubulin isotype 3. Brain 2013, 136, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Al-Mehdi, A.B.; Pastukh, V.M.; Swiger, B.M.; Reed, D.J.; Patel, M.R.; Bardwell, G.C.; Pastukh, V.V.; Alexeyev, M.F.; Gillespie, M.N. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci. Signal. 2012, 5, ra47. [Google Scholar] [CrossRef] [PubMed]
- Goyal, U.; Blackstone, C. Untangling the web: Mechanisms underlying ER network formation. Biochim. Biophys. Acta 2013, 1833, 2492–2498. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Chen, L.B.; Fujiwara, K. Microtubules and the endoplasmic-reticulum are highly interdependent structures. J. Cell Biol. 1986, 103, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Waterman-Storer, C.M.; Salmon, E.D. Microtubule dynamics: Treadmilling comes around again. Curr. Biol. 1997, 7, R369–R372. [Google Scholar] [CrossRef]
- Friedman, J.R.; Webster, B.M.; Mastronarde, D.N.; Verhey, K.J.; Voeltz, G.K. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J. Cell Biol. 2010, 190, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Chen, L.B. Dynamic behavior of endoplasmic-reticulum in living cells. Cell 1988, 54, 37–46. [Google Scholar] [CrossRef]
- Chen, G.A.; Gharib, T.G.; Wang, H.; Huang, C.C.; Kuick, R.; Thomas, D.G.; Shedden, K.A.; Misek, D.E.; Taylor, J.M.G.; Giordano, T.J.; et al. Protein profiles associated with survival in lung adenocarcinoma. Proc. Natl. Acad. Sci. USA 2003, 100, 13537–13542. [Google Scholar] [CrossRef] [PubMed]
- Waterman-Storer, C.M.; Salmon, E.D. Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms. Curr. Biol. 1998, 8, 798. [Google Scholar] [CrossRef]
- Sriburi, R.; Jackowski, S.; Mori, K.; Brewer, J.W. XBP1: A link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J. Cell Biol. 2004, 167, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Schuck, S.; Prinz, W.A.; Thorn, K.S.; Voss, C.; Walter, P. Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J. Cell Biol. 2009, 187, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, R.; Ma, N.; Xiao, H.B.; Chen, Y.; Chen, F.; Mei, J.; Ding, F.B.; Zhong, H. Phosphorylation of eIF2α suppresses cisplatin-induced A549 cell apoptosis via p38 inhibition. Cancer Biother. Radiopharm. 2013, 28, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Hua, J.; Wang, Q.; Xu, W.; Zhang, J.X.; Zhang, J.; Kang, J.H.; Li, M.Q. Expressions of GRP78 and Bax associate with differentiation, metastasis, and apoptosis in non-small cell lung cancer. Mol. Biol. Rep. 2012, 39, 6753–6761. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kaufman, R.J. The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer 2014, 14, 581–597. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Y.; Xia, B.; White, E. Autophagy-mediated tumor promotion. Cell 2013, 155, 1216–1219. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Chen, R.; Wang, Z.; Huang, Z.; Kong, N.; Zhang, M.; Han, W.; Lou, F.; Yang, J.; Zhang, Q.; et al. Autophagy and chemotherapy resistance: A promising therapeutic target for cancer treatment. Cell Death Dis. 2013, 4, e838. [Google Scholar] [CrossRef] [PubMed]
- Macintosh, R.L.; Ryan, K.M. Autophagy in tumour cell death. Semin. Cancer Biol. 2013, 23, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, Y.; Ohashi, R.; Kawamura, T.; Iwanari, H.; Kodama, T.; Naito, M.; Hamakubo, T. Phosphatidylinositol 3-phosphatase myotubularin-related protein 6 (MTMR6) is regulated by small GTPase Rab1B in the early secretory and autophagic pathways. J. Biol. Chem. 2013, 288, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Sancak, Y.; Bar-Peled, L.; Zoncu, R.; Markhard, A.L.; Nada, S.; Sabatini, D.M. Ragulator-rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010, 141, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Geeraert, C.; Ratier, A.; Pfisterer, S.G.; Perdiz, D.; Cantaloube, I.; Rouault, A.; Pattingre, S.; Proikas-Cezanne, T.; Codogno, P.; Pous, C. Starvation-induced hyperacetylation of tubulin is required for the stimulation of autophagy by nutrient deprivation. J. Biol. Chem. 2010, 285, 24184–24194. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, S.; Corazzari, M.; Nazio, F.; Oliverio, S.; Lisi, G.; Antonioli, M.; Pagliarini, V.; Matteoni, S.; Fuoco, C.; Giunta, L.; et al. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J. Cell Biol. 2010, 191, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.Q.; Garcia-Arencibia, M.; Zhao, R.; Puri, C.; Toh, P.P.C.; Sadiq, O.; Rubinsztein, D.C. Bim inhibits autophagy by recruiting beclin 1 to microtubules. Mol. Cell 2012, 47, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Noda, T.; Yoshimori, T. Dynein-dependent movement of autophagosomes mediates efficient encounters with lysosomes. Cell Struct. Funct. 2008, 33, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Kochl, R.; Hu, X.W.; Chan, E.Y.W.; Tooze, S.A. Microtubules facilitate autophagosome formation and fusion of autophagosomes with endosomes. Traffic 2006, 7, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Amenta, J.S.; Sargus, M.J.; Baccino, F.M. Effect of microtubular or translational inhibitors on general cell protein degradation—Evidence for a dual catabolic pathway. Biochem. J. 1977, 168, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Aplin, A.; Jasionowski, T.; Tuttle, D.L.; Lenk, S.E.; Dunn, W.A. Cytoskeletal elements are required for the formation and maturation of autophagic vacuoles. J. Cell. Physiol. 1992, 152, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Reunanen, H.; Marttinen, M.; Hirsimaki, P. Effects of griseofulvin and nocodazole on the accumulation of autophagic vacuoles in ehrlich ascites tumor-cells. Exp. Mol. Pathol. 1988, 48, 97–102. [Google Scholar] [CrossRef]
- Kraft, L.J.; Manral, P.; Dowler, J.; Kenworthy, A.K. Nuclear LC3 associates with slowly diffusing complexes that survey the nucleolus. Traffic 2016, 17, 369–399. [Google Scholar] [CrossRef] [PubMed]
- Vilmar, A.C.; Santoni-Rugiu, E.; Sorensen, J.B. Class III β-tubulin in advanced NSCLC of adenocarcinoma subtype predicts superior outcome in a randomized trial. Clin. Cancer Res. 2011, 17, 5205–5214. [Google Scholar] [CrossRef] [PubMed]
- Katsetos, C.D.; Del Valle, L.; Geddes, J.F.; Aldape, K.; Boyd, J.C.; Legido, A.; Khalili, K.; Perentes, E.; Mork, S.J. Localization of the neuronal class III β-tubulin in oligodendrogliomas: Comparison with Ki-67 proliferative index and 1p/19q status. J. Neuropathol. Exp. Neurol. 2002, 61, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Katsetos, C.D.; Draberova, E.; Smejkalova, B.; Reddy, G.; Bertrand, L.; de Chadarevian, J.P.; Legido, A.; Nissanov, J.; Baas, P.W.; Draber, P. Class III β-tubulin and gamma-tubulin are co-expressed and form complexes in human glioblastoma cells. Neurochem. Res. 2007, 32, 1387–1398. [Google Scholar] [CrossRef] [PubMed]
- Egevad, L.; Valdman, A.; Wiklund, N.P.; Seve, P.; Dumontet, C. β-tubulin III expression in prostate cancer. Scand. J. Urol. Nephrol. 2010, 44, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Katsetos, C.D.; Kontogeorgos, G.; Geddes, J.F.; Herman, M.M.; Tsimara-Papastamatiou, H.; Yu, Y.X.; Sakkas, L.I.; Tsokos, M.; Patchefsky, A.S.; Ehya, H.; et al. Differential distribution of the neuron-associated class III β-tubulin in neuroendocrine lung tumors. Arch. Pathol. Lab. Med. 2000, 124, 535–544. [Google Scholar] [PubMed]
- Seve, P.; Dumontet, C. Is class III β-tubulin a predictive factor in patients receiving tubulin-binding agents? Lancet Oncol. 2008, 9, 168–175. [Google Scholar] [CrossRef]
- Zhao, X.; Yue, C.; Chen, J.; Tian, C.; Yang, D.; Xing, L.; Liu, H.; Jin, Y. Class III β-tubulin in colorectal cancer: Tissue distribution and clinical analysis of chinese patients. Med. Sci. Monit. 2016, 22, 3915–3924. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, S.; He, S.; Kopf, M.; Mariani, M.; Petrini, J.; Scambia, G.; Ferlini, C. Free testosterone drives cancer aggressiveness: Evidence from us population studies. PLoS ONE 2013, 8, e61955. [Google Scholar] [CrossRef] [PubMed]
- Orsted, D.D.; Nordestgaard, B.G.; Bojesen, S.E. Plasma testosterone in the general population, cancer prognosis and cancer risk: A prospective cohort study. Ann. Oncol. 2014, 25, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Katsetos, C.D.; Herman, M.M.; Mork, S.J. Class III β-tubulin in human development and cancer. Cell Motil. Cytoskelet. 2003, 55, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Mi, R.; Pan, C.; Zhou, Y.; Liu, Y.; Jin, G.; Liu, F. Identification of the metastasis potential and its associated genes in melanoma multinucleated giant cells using the PHA-ECM830 fusion method. Oncol. Rep. 2016, 35, 211–218. [Google Scholar] [CrossRef] [PubMed]
- He, Z.Y.; Wen, H.; Shi, C.B.; Wang, J. Up-regulation of hnRNP A1, Ezrin, tubulin β-2C and Annexin A1 in sentinel lymph nodes of colorectal cancer. World J. Gastroenterol. 2010, 16, 4670–4676. [Google Scholar] [CrossRef] [PubMed]
- Atjanasuppat, K.; Lirdprapamongkol, K.; Jantaree, P.; Svasti, J. Non-adherent culture induces paclitaxel resistance in H460 lung cancer cells via ERK-mediated up-regulation of βIVa-tubulin. Biochem. Biophys. Res. Commun. 2015, 466, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Sobierajska, K.; Wieczorek, K.; Ciszewski, W.M.; Sacewicz-Hofman, I.; Wawro, M.E.; Wiktorska, M.; Boncela, J.; Papiewska-Pajak, I.; Kwasniak, P.; Wyroba, E.; et al. β-III tubulin modulates the behavior of snail overexpressed during the epithelial-to-mesenchymal transition in colon cancer cells. Biochim. Biophys. Acta 2016, 1863, 2221–2233. [Google Scholar] [CrossRef] [PubMed]

| Alteration of Tubulin Isotype | Effect | Tumour Type | Reference |
|---|---|---|---|
| High βI-tubulin | Poor response to docetaxel treatment | Breast cancer | [17] |
| Low βII-tubulin expression | Correlates with poor response to taxane treatment or advanced stage disease | Breast and ovarian cancer | [19,22] |
| High βIII-tubulin expression | Poor survival, poor outcome for surgical resection or TBA response; Correlates with subtype | Non-small cell lung cancer (NSCLC) | [24,25,37,38,39,40,41,42,43,44,45] |
| Correlates with poor survival, poor response to platinum and taxane treatment, advanced stage or aggressive disease | Ovarian cancer | [19,20,21,28,29,30,31,32,33] | |
| Favourable response to taxane treatment | Ovarian (Clear cell adenocarcinoma) | [34] | |
| Poor response to taxane treatment | Breast cancer | [17,46] | |
| Poor response to taxane/platinum treatment | Uterine serous carcinoma | [18] | |
| Poor response to taxane treatment | Gastric cancer | [26,47] | |
| Advanced disease and early recurrence | Prostate cancer | [48] | |
| High βIVa-tubulin expression | Poor response to taxol treatment | Ovarian cancer | [29] |
| High βV-tubulin expression | Favourable response to taxane treatment | NSCLC | [27] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parker, A.L.; Teo, W.S.; McCarroll, J.A.; Kavallaris, M. An Emerging Role for Tubulin Isotypes in Modulating Cancer Biology and Chemotherapy Resistance. Int. J. Mol. Sci. 2017, 18, 1434. https://doi.org/10.3390/ijms18071434
Parker AL, Teo WS, McCarroll JA, Kavallaris M. An Emerging Role for Tubulin Isotypes in Modulating Cancer Biology and Chemotherapy Resistance. International Journal of Molecular Sciences. 2017; 18(7):1434. https://doi.org/10.3390/ijms18071434
Chicago/Turabian StyleParker, Amelia L., Wee Siang Teo, Joshua A. McCarroll, and Maria Kavallaris. 2017. "An Emerging Role for Tubulin Isotypes in Modulating Cancer Biology and Chemotherapy Resistance" International Journal of Molecular Sciences 18, no. 7: 1434. https://doi.org/10.3390/ijms18071434
APA StyleParker, A. L., Teo, W. S., McCarroll, J. A., & Kavallaris, M. (2017). An Emerging Role for Tubulin Isotypes in Modulating Cancer Biology and Chemotherapy Resistance. International Journal of Molecular Sciences, 18(7), 1434. https://doi.org/10.3390/ijms18071434