Abnormally Increased Secretion in Olfactory Neuronal Precursors from a Case of Schizophrenia Is Modulated by Melatonin: A Pilot Study
Abstract
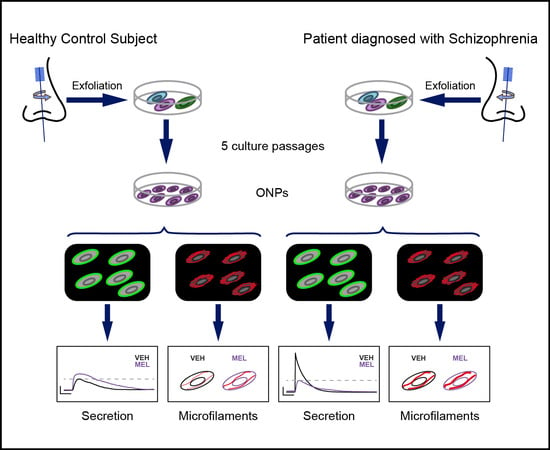
:1. Introduction
2. Results
2.1. Characterization of Secretory Vesicles and of Potassium-Evoked Calcium Dependent Secretion in Human Olfactory Neuronal Precursors (ONPs)
2.1.1. Vesicle-Associated Membrane Protein 1/2 (VAMP1/2)-Positive Secretory Vesicles are Present in Human ONPs
2.1.2. Characterization of Potassium-Evoked Secretion in ONPs
2.2. Comparison of Extrasynaptic Secretion from HCS- and SCZ-Derived ONPs and the Effects of Melatonin on Secretion
2.3. Evaluation of VAMP1/2-Immunofluorescence Intensity in HCS and SCZ-Derived ONPs
2.4. Evaluation of Actin Microfilament Thickness in HCS- and SCZ-Derived ONPs
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Primary Cell Culture
4.3. Immunofluorescence
4.4. Analysis of Secretion in Olfactory Neuronal Precursors
4.5. Actin Microfilaments Fluorescent Staining
4.6. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Joseph, J.; Kremen, W.S.; Franz, C.E.; Glatt, S.J.; van de Leemput, J.; Chandler, S.D.; Tsuang, M.T.; Twamley, E.W. Predictors of current functioning and functional decline in schizophrenia. Schizophr. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Primers 2015, 1, 15067. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Saha, S.; Chant, D.; Welham, J. Schizophrenia: A concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 2008, 30, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Marder, S.R.; Galderisi, S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry 2017, 16, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Chiapponi, C.; Piras, F.; Caltagirone, C.; Spalletta, G. GABA System in Schizophrenia and Mood Disorders: A Mini Review on Third-Generation Imaging Studies. Front Psychiatry 2016, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Iasevoli, F.; Tomasetti, C.; Buonaguro, E.F.; de Bartolomeis, A. The glutamatergic aspects of schizophrenia molecular pathophysiology: Role of the postsynaptic density, and implications for treatment. Curr. Neuropharmacol. 2014, 12, 219–238. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Meyer-Lindenberg, A. Striatal presynaptic dopamine in schizophrenia, part II: Meta-analysis of [18F/11C]-DOPA PET studies. Schizophr. Bull. 2013, 39, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Breier, A.; Su, T.P.; Saunders, R.; Carson, R.E.; Kolachana, B.S.; de Bartolomeis, A.; Weinberger, D.R.; Weisenfeld, N.; Malhotra, A.K.; Eckelman, W.C.; et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: Evidence from a novel positron emission tomography method. Proc. Natl. Acad. Sci. USA 1997, 94, 2569–2574. [Google Scholar] [CrossRef] [PubMed]
- Abi-Dargham, A.; Gil, R.; Krystal, J.; Baldwin, R.M.; Seibyl, J.P.; Bowers, M.; van Dyck, C.H.; Charney, D.S.; Innis, R.B.; Laruelle, M. Increased striatal dopamine transmission in schizophrenia: Confirmation in a second cohort. Am. J. Psychiatry 1998, 155, 761–777. [Google Scholar] [PubMed]
- Laruelle, M.; Abi-Dargham, A.; Gil, R.; Kegeles, L.; Innis, R. Increased dopamine transmission in schizophrenia: Relationship to illness phases. Biol. Psychiatry 1999, 46, 56–72. [Google Scholar] [CrossRef]
- Hook, V.; Brennand, K.J.; Kim, Y.; Toneff, T.; Funkelstein, L.; Lee, K.C.; Ziegler, M.; Gage, F.H. Human iPSC neurons display activity-dependent neurotransmitter secretion: Aberrant catecholamine levels in schizophrenia neurons. Stem Cell. Rep. 2014, 3, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Trueta, C.; de-Miguel, F.F. Extrasynaptic exocytosis and its mechanisms: A source of molecules mediating volume transmission in the nervous system. Front. Physiol. 2012, 3, 319. [Google Scholar] [CrossRef] [PubMed]
- Porat-Shliom, N.; Milberg, O.; Masedunskas, A.; Weigert, R. Multiple roles for the actin cytoskeleton during regulated exocytosis. Cell. Mol. Life Sci. 2013, 70, 2099–2121. [Google Scholar] [CrossRef] [PubMed]
- Bellon, A.; Ortiz-Lopez, L.; Ramirez-Rodriguez, G.; Anton-Tay, F.; Benitez-King, G. Melatonin induces neuritogenesis at early stages in N1E-115 cells through actin rearrangements via activation of protein kinase C and Rho-associated kinase. J. Pineal Res. 2007, 42, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Benitez-King, G. Melatonin as a cytoskeletal modulator: Implications for cell physiology and disease. J. Pineal Res. 2006, 40, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Galvan-Arrieta, T.; Trueta, C.; Cercos, M.G.; Valdes-Tovar, M.; Alarcon, S.; Oikawa, J.; Zamudio-Meza, H.; Benitez-King, G. The role of melatonin in the neurodevelopmental etiology of schizophrenia: A study in human olfactory neuronal precursors. J. Pineal Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Benitez-King, G.; Valdes-Tovar, M.; Trueta, C.; Galvan-Arrieta, T.; Argueta, J.; Alarcon, S.; Lora-Castellanos, A.; Solis-Chagoyan, H. The microtubular cytoskeleton of olfactory neurons derived from patients with schizophrenia or with bipolar disorder: Implications for biomarker characterization, neuronal physiology and pharmacological screening. Mol. Cell. Neurosci. 2016, 73, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Somera-Molina, K.C.; Hudson, R.L.; Dubocovich, M.L. Melatonin potentiates running wheel-induced neurogenesis in the dentate gyrus of adult C3H/HeN mice hippocampus. J. Pineal Res. 2013, 54, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, G.; Ortiz-Lopez, L.; Dominguez-Alonso, A.; Benitez-King, G.A.; Kempermann, G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J. Pineal Res. 2011, 50, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Alonso, A.; Ramirez-Rodriguez, G.; Benitez-King, G. Melatonin increases dendritogenesis in the hilus of hippocampal organotypic cultures. J. Pineal Res. 2012, 52, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Goodall, A.R.; Danks, K.; Walker, J.H.; Ball, S.G.; Vaughan, P.F. Occurrence of two types of secretory vesicles in the human neuroblastoma SH-SY5Y. J. Neurochem. 1997, 68, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Betz, W.J.; Bewick, G.S. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science 1992, 255, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Dancker, P.; Low, I.; Hasselbach, W.; Wieland, T. Interaction of actin with phalloidin: Polymerization and stabilization of F-actin. Biochim. Biophys. Acta 1975, 400, 407–414. [Google Scholar] [CrossRef]
- Egbujo, C.N.; Sinclair, D.; Hahn, C.G. Dysregulations of Synaptic Vesicle Trafficking in Schizophrenia. Curr. Psychiatry Rep. 2016, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Benitez-King, G.; Riquelme, A.; Ortiz-Lopez, L.; Berlanga, C.; Rodriguez-Verdugo, M.S.; Romo, F.; Calixto, E.; Solis-Chagoyan, H.; Jimenez, M.; et al. A non-invasive method to isolate the neuronal linage from the nasal epithelium from schizophrenic and bipolar diseases. J. Neurosci. Methods 2011, 201, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Matigian, N.; Abrahamsen, G.; Sutharsan, R.; Cook, A.L.; Vitale, A.M.; Nouwens, A.; Bellette, B.; An, J.; Anderson, M.; Beckhouse, A.G.; et al. Disease-specific, neurosphere-derived cells as models for brain disorders. Dis. Models Mech. 2010, 3, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Abi-Dargham, A.; van de Giessen, E.; Slifstein, M.; Kegeles, L.S.; Laruelle, M. Baseline and amphetamine-stimulated dopamine activity are related in drug-naive schizophrenic subjects. Biol. Psychiatry 2009, 65, 1091–1093. [Google Scholar] [CrossRef] [PubMed]
- Laruelle, M.; Abi-Dargham, A. Dopamine as the wind of the psychotic fire: New evidence from brain imaging studies. J. Psychopharmacol. 1999, 13, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Lyon, G.J.; Abi-Dargham, A.; Moore, H.; Lieberman, J.A.; Javitch, J.A.; Sulzer, D. Presynaptic regulation of dopamine transmission in schizophrenia. Schizophr. Bull. 2011, 37, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sanchez, N.; Rodriguez-Rodriguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L. Melatonin is a potent modulator of dopamine release in the retina. Nature 1983, 306, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Zisapel, N.; Laudon, M. Inhibition by melatonin of dopamine release from rat hypothalamus: Regulation of calcium entry. Brain Res. 1983, 272, 378–381. [Google Scholar] [CrossRef]
- Zisapel, N.; Egozi, Y.; Laudon, M. Inhibition of dopamine release by melatonin: Regional distribution in the rat brain. Brain Res. 1982, 246, 161–163. [Google Scholar] [CrossRef]
- Monteleone, P.; Natale, M.; La Rocca, A.; Maj, M. Decreased nocturnal secretion of melatonin in drug-free schizophrenics: No change after subchronic treatment with antipsychotics. Neuropsychobiology 1997, 36, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Schoch, S.; Deak, F.; Konigstorfer, A.; Mozhayeva, M.; Sara, Y.; Sudhof, T.C.; Kavalali, E.T. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science 2001, 294, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sugiura, Y.; Lin, W. The role of synaptobrevin1/VAMP1 in Ca2+-triggered neurotransmitter release at the mouse neuromuscular junction. J. Physiol. 2011, 589, 1603–1618. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Tanaka, H.; Wakabayashi, C.; Yamamoto, N.; Uchiyama, H.; Teraishi, T.; Hori, H.; Arima, K.; Kunugi, H. Expression of Ca2+-dependent activator protein for secretion 2 is increased in the brains of schizophrenic patients. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1738–1743. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, S.; Atluri, P.P.; Ryan, T.A. Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat. Neurosci. 2003, 6, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Gasman, S.; Chasserot-Golaz, S.; Malacombe, M.; Way, M.; Bader, M.F. Regulated exocytosis in neuroendocrine cells: A role for subplasmalemmal Cdc42/N-WASP-induced actin filaments. Mol. Biol. Cell. 2004, 15, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Jung, Y.H.; Oh, S.Y.; Yun, S.P.; Han, H.J. Melatonin enhances the human mesenchymal stem cells motility via melatonin receptor 2 coupling with Galphaq in skin wound healing. J. Pineal Res. 2014, 57, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Dinno, A. Dunn’s Test of Multiple Comparissons Using Rank Sums. R Package Version 1.3.4. Available online: https://CRAN.R-project.org/package=dunn.test (accessed on 2 April 2017).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org (accessed on 29 March 2017).




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cercós, M.G.; Galván-Arrieta, T.; Valdés-Tovar, M.; Solís-Chagoyán, H.; Argueta, J.; Benítez-King, G.; Trueta, C. Abnormally Increased Secretion in Olfactory Neuronal Precursors from a Case of Schizophrenia Is Modulated by Melatonin: A Pilot Study. Int. J. Mol. Sci. 2017, 18, 1439. https://doi.org/10.3390/ijms18071439
Cercós MG, Galván-Arrieta T, Valdés-Tovar M, Solís-Chagoyán H, Argueta J, Benítez-King G, Trueta C. Abnormally Increased Secretion in Olfactory Neuronal Precursors from a Case of Schizophrenia Is Modulated by Melatonin: A Pilot Study. International Journal of Molecular Sciences. 2017; 18(7):1439. https://doi.org/10.3390/ijms18071439
Chicago/Turabian StyleCercós, Montserrat G., Tania Galván-Arrieta, Marcela Valdés-Tovar, Héctor Solís-Chagoyán, Jesús Argueta, Gloria Benítez-King, and Citlali Trueta. 2017. "Abnormally Increased Secretion in Olfactory Neuronal Precursors from a Case of Schizophrenia Is Modulated by Melatonin: A Pilot Study" International Journal of Molecular Sciences 18, no. 7: 1439. https://doi.org/10.3390/ijms18071439
APA StyleCercós, M. G., Galván-Arrieta, T., Valdés-Tovar, M., Solís-Chagoyán, H., Argueta, J., Benítez-King, G., & Trueta, C. (2017). Abnormally Increased Secretion in Olfactory Neuronal Precursors from a Case of Schizophrenia Is Modulated by Melatonin: A Pilot Study. International Journal of Molecular Sciences, 18(7), 1439. https://doi.org/10.3390/ijms18071439