Relationship between Aging-Related Skin Dryness and Aquaporins
Abstract
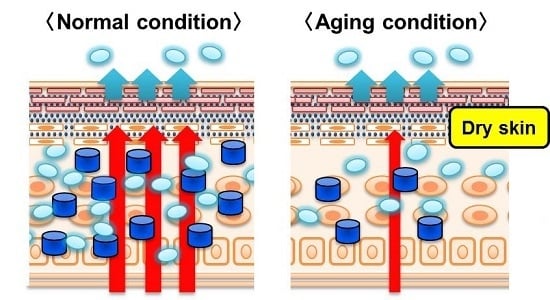
:1. Introduction
2. Results
2.1. Body Weight, Transepidermal Water Loss (TEWL) and Dermal Water Content
2.2. The Expression Level of Aquaporins (AQPs) in the Skin
2.3. The Expression Level of AQPs in the Whole Body
3. Discussion
4. Materials and Methods
4.1. Animals and Treatments
4.2. Measurement of TEWL and Dermal Water Content
4.3. Real-Time RT-PCR
4.4. Preparation of Membrane Fraction from Skin for Immunoblotting
4.5. Electrophoresis and Immunoblotting
4.6. Immunohistochemistry
4.7. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AQPs | aquaporins |
| TEWL | transepidermal water loss |
| PBS | phosphate-buffered saline |
References
- Ishibashi, K.; Hara, S.; Kondo, S. Aquaporin water channels in mammals. Clin. Exp. Nephrol. 2009, 13, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Rojek, A.; Fuchtbauer, E.M.; Kwon, T.H.; Frokiaer, J.; Nielsen, S. Severe urinary concentrating defect in renal collecting duct-selective AQP2 conditional-knockout mice. Proc. Natl. Acad. Sci. USA 2006, 103, 6037–6042. [Google Scholar] [CrossRef] [PubMed]
- Binder, D.K.; Oshio, K.; Ma, T.; Verkman, A.S.; Manley, G.T. Increased seizure threshold in mice lacking aquaporin-4 water channels. Neuroreport 2004, 15, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Manley, G.T.; Binder, D.K.; Papadopoulos, M.C.; Verkman, A.S. New insights into water transport and edema in the central nervous system from phenotype analysis of aquaporin-4 null mice. Neuroscience 2004, 129, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, N.; Baba, K.; Ushiki, T.; Kon, R.; Mimura, A.; Toda, T.; Ishii, M.; Ochiai, W.; Sugiyama, K. The laxative effect of bisacodyl is attributable to decreased aquaporin-3 expression in the colon induced by increased PGE2 secretion from macrophages. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 301, G887–G895. [Google Scholar] [CrossRef] [PubMed]
- Kon, R.; Ikarashi, N.; Hayakawa, A.; Haga, Y.; Fueki, A.; Kusunoki, Y.; Tajima, M.; Ochiai, W.; Machida, Y.; Sugiyama, K. Morphine-Induced Constipation Develops With Increased Aquaporin-3 Expression in the Colon via Increased Serotonin Secretion. Toxicol. Sci. Off. J. Soc. Toxicol. 2015, 145, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Kon, R.; Ikarashi, N.; Nagoya, C.; Takayama, T.; Kusunoki, Y.; Ishii, M.; Ueda, H.; Ochiai, W.; Machida, Y.; Sugita, K.; et al. Rheinanthrone, a metabolite of sennoside A, triggers macrophage activation to decrease aquaporin-3 expression in the colon, causing the laxative effect of rhubarb extract. J. Ethnopharmacol. 2014, 152, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Boury-Jamot, M.; Sougrat, R.; Tailhardat, M.; Le Varlet, B.; Bonte, F.; Dumas, M.; Verbavatz, J.M. Expression and function of aquaporins in human skin: Is aquaporin-3 just a glycerol transporter? Biochim. Biophys. Acta 2006, 1758, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Sougrat, R.; Morand, M.; Gondran, C.; Barre, P.; Gobin, R.; Bonte, F.; Dumas, M.; Verbavatz, J.M. Functional expression of AQP3 in human skin epidermis and reconstructed epidermis. J. Investig. Dermatol. 2002, 118, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Aquaporin-3 functions as a glycerol transporter in mammalian skin. Biol. Cell 2005, 97, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Hara, M.; Sougrat, R.; Verbavatz, J.M.; Verkman, A.S. Impaired stratum corneum hydration in mice lacking epidermal water channel aquaporin-3. J. Biol. Chem. 2002, 277, 17147–17153. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Ma, T.; Verkman, A.S. Selectively reduced glycerol in skin of aquaporin-3-deficient mice may account for impaired skin hydration, elasticity, and barrier recovery. J. Biol. Chem. 2002, 277, 46616–46621. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Zheng, X.; Zhong, X.; Shetty, A.K.; Elias, P.M.; Bollag, W.B. Aquaporin-3 in keratinocytes and skin: Its role and interaction with phospholipase D2. Arch. Biochem. Biophys. 2011, 508, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Verkman, A.S. Aquaporin-3 facilitates epidermal cell migration and proliferation during wound healing. J. Mol. Med. 2008, 86, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Kevin Heard, L.; Chen, X.; Bollag, W.B. Aquaporins in the Skin. Adv. Exp. Med. Biol. 2017, 969, 173–191. [Google Scholar] [PubMed]
- Ghadially, R.; Brown, B.E.; Sequeira-Martin, S.M.; Feingold, K.R.; Elias, P.M. The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model. J. Clin. Investig. 1995, 95, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Ghersetich, I.; Lotti, T.; Campanile, G.; Grappone, C.; Dini, G. Hyaluronic acid in cutaneous intrinsic aging. Int. J. Dermatol. 1994, 33, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.M.; Forl, M.; Winoto-Morbach, S.; Seite, S.; Schunck, M.; Proksch, E.; Schutze, S. Acid and neutral sphingomyelinase, ceramide synthase, and acid ceramidase activities in cutaneous aging. Exp. Dermatol. 2005, 14, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Oliver, N.; Sternlicht, M.; Gerritsen, K.; Goldschmeding, R. Could aging human skin use a connective tissue growth factor boost to increase collagen content? J. Investig. Dermatol. 2010, 130, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, C.; Kierbel, A.; Amodeo, G.; Zotta, E.; Bigi, F.; Berkowski, D.; Ibarra, C. Functional characterization and localization of AQP3 in the human colon. Braz. J. Med. Biol. Res. 1999, 32, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Spector, D.A.; Wade, J.B.; Dillow, R.; Steplock, D.A.; Weinman, E.J. Expression, localization, and regulation of aquaporin-1 to -3 in rat urothelia. Am. J. Physiol. Ren. Physiol. 2002, 282, F1034–F1042. [Google Scholar] [CrossRef] [PubMed]
- Baumgarten, R.; Van De Pol, M.H.; Wetzels, J.F.; Van Os, C.H.; Deen, P.M. Glycosylation is not essential for vasopressin-dependent routing of aquaporin-2 in transfected Madin-Darby canine kidney cells. J. Am. Soc. Nephrol. 1998, 9, 1553–1559. [Google Scholar] [PubMed]
- Hendriks, G.; Koudijs, M.; van Balkom, B.W.; Oorschot, V.; Klumperman, J.; Deen, P.M.; van der Sluijs, P. Glycosylation is important for cell surface expression of the water channel aquaporin-2 but is not essential for tetramerization in the endoplasmic reticulum. J. Biol. Chem. 2004, 279, 2975–2983. [Google Scholar] [CrossRef] [PubMed]
- Umenishi, F.; Narikiyo, T.; Schrier, R.W. Effect on stability, degradation, expression, and targeting of aquaporin-2 water channel by hyperosmolality in renal epithelial cells. Biochem. Biophys. Res. Commun. 2005, 338, 1593–1599. [Google Scholar] [CrossRef] [PubMed]
- Ferando, I.; Faas, G.C.; Mody, I. Diminished KCC2 confounds synapse specificity of LTP during senescence. Nat. Neurosci. 2016, 19, 1197–1200. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, N.; Sato, W.; Toda, T.; Ishii, M.; Ochiai, W.; Sugiyama, K. Inhibitory Effect of Polyphenol-Rich Fraction from the Bark of Acacia mearnsii on Itching Associated with Allergic Dermatitis. Evid Based Complement. Alternat. Med. 2012, 2012, 120389. [Google Scholar] [CrossRef] [PubMed]
- Nejsum, L.N.; Kwon, T.H.; Jensen, U.B.; Fumagalli, O.; Frokiaer, J.; Krane, C.M.; Menon, A.G.; King, L.S.; Agre, P.C.; Nielsen, S. Functional requirement of aquaporin-5 in plasma membranes of sweat glands. Proc. Natl. Acad. Sci. USA 2002, 99, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Hara-Chikuma, M.; Sugiyama, Y.; Kabashima, K.; Sohara, E.; Uchida, S.; Sasaki, S.; Inoue, S.; Miyachi, Y. Involvement of aquaporin-7 in the cutaneous primary immune response through modulation of antigen uptake and migration in dendritic cells. FASEB J. 2012, 26, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, T.; Lagerholm, B.C.; Vikstrom, E.; Loitto, V.M.; Magnusson, K.E. Water fluxes through aquaporin-9 prime epithelial cells for rapid wound healing. Biochem. Biophys. Res. Commun. 2013, 430, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Loitto, V.M.; Huang, C.; Sigal, Y.J.; Jacobson, K. Filopodia are induced by aquaporin-9 expression. Exp. Cell Res. 2007, 313, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Preisser, L.; Teillet, L.; Aliotti, S.; Gobin, R.; Berthonaud, V.; Chevalier, J.; Corman, B.; Verbavatz, J.M. Downregulation of aquaporin-2 and -3 in aging kidney is independent of V2 vasopressin receptor. Am. J. Physiol. Ren. Physiol. 2000, 279, F144–F152. [Google Scholar]
- Korlimbinis, A.; Berry, Y.; Thibault, D.; Schey, K.L.; Truscott, R.J. Protein aging: Truncation of aquaporin 0 in human lens regions is a continuous age-dependent process. Exp. Eye Res. 2009, 88, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Bellemere, G.; Von Stetten, O.; Oddos, T. Retinoic acid increases aquaporin 3 expression in normal human skin. J. Investig. Dermatol. 2008, 128, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Dumas, M.; Sadick, N.S.; Noblesse, E.; Juan, M.; Lachmann-Weber, N.; Boury-Jamot, M.; Sougrat, R.; Verbavatz, J.M.; Schnebert, S.; Bonte, F. Hydrating skin by stimulating biosynthesis of aquaporins. J. Drugs Dermatol. 2007, 6, s20–s24. [Google Scholar] [PubMed]
- Jiang, Y.J.; Kim, P.; Lu, Y.F.; Feingold, K.R. PPARγ activators stimulate aquaporin 3 expression in keratinocytes/epidermis. Exp. Dermatol. 2011, 20, 595–599. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward | Reverse |
|---|---|---|
| AQP0 | TGCTCTGCATCTTTGCTACA | GCACCAGTGTAATACATCCCA |
| AQP1 | CTGCTGGCGATTGACTACACT | TCATAGATGAGCACTGCCAGG |
| AQP2 | CTGGCTGTCAATGCTCTCCAC | TTGTCACTGCGGCGCTCATC |
| AQP3 | CCTTGTGATGTTTGGCTGTGG | GGAAGCACATTGCGAAGGTC |
| AQP4 | GAGTCACCACGGTTCATGGA | CGTTTGGAATCACAGCTGGC |
| AQP5 | GCTGGAGAGGCAGCATTG | CACCCAAGTGTCCCATCATG |
| AQP7 | GCTTGGTCTGCTGCTTCAG | GGAACTCTGCCAGAAACTCTC |
| AQP8 | GGCTTCTCTGTCATTGTGGA | TCCGATGAGGAGCCTAATGA |
| AQP9 | TGAGCCATTAGGAGAGACCTT | ACCTCCAACTTTAGTCCACCA |
| β-actin | GAGCGCAAGTACTCTGTGTG | CGGACTCATCGTACTCCTG |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikarashi, N.; Kon, R.; Kaneko, M.; Mizukami, N.; Kusunoki, Y.; Sugiyama, K. Relationship between Aging-Related Skin Dryness and Aquaporins. Int. J. Mol. Sci. 2017, 18, 1559. https://doi.org/10.3390/ijms18071559
Ikarashi N, Kon R, Kaneko M, Mizukami N, Kusunoki Y, Sugiyama K. Relationship between Aging-Related Skin Dryness and Aquaporins. International Journal of Molecular Sciences. 2017; 18(7):1559. https://doi.org/10.3390/ijms18071559
Chicago/Turabian StyleIkarashi, Nobutomo, Risako Kon, Miho Kaneko, Nanaho Mizukami, Yoshiki Kusunoki, and Kiyoshi Sugiyama. 2017. "Relationship between Aging-Related Skin Dryness and Aquaporins" International Journal of Molecular Sciences 18, no. 7: 1559. https://doi.org/10.3390/ijms18071559
APA StyleIkarashi, N., Kon, R., Kaneko, M., Mizukami, N., Kusunoki, Y., & Sugiyama, K. (2017). Relationship between Aging-Related Skin Dryness and Aquaporins. International Journal of Molecular Sciences, 18(7), 1559. https://doi.org/10.3390/ijms18071559