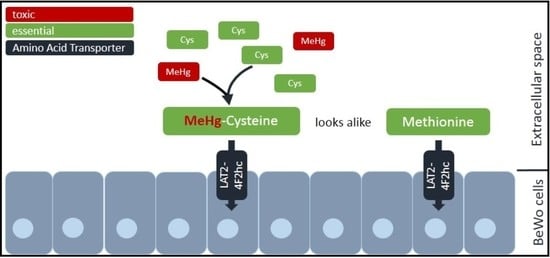
Methylmercury Uptake into BeWo Cells Depends on LAT2-4F2hc, a System L Amino Acid Transporter
Abstract
:1. Introduction
2. Results
2.1. Pre-Experiments
2.2. Forskolin-Induced BeWo Cell Fusion Increases LAT1 and LAT2 Expression
2.3. LAT2 and 4F2hc Downregulation Reduces Mercury Uptake into BeWo Cells
2.4. LAT2 and 4F2hc Downregulation Reduces Methionine and Leucine Uptake into BeWo Cells
3. Discussion
3.1. Dose and Time Dependent Uptake into BeWo Cells
3.2. LAT1, LAT2, and 4F2hc Downregulation Does Not Affect BeWo Cell Number
3.3. Forskolin-Induced BeWo Cell Fusion Increases LAT1 and LAT2 Expression
3.4. LAT2 and 4F2hc Downregulation Reduces Uptake of Methylmercury, Leucine and Methionine into BeWo Cells
3.5. Uptake of MeHg, Leucine and Methionine in Relation to Transporter Localization
4. Materials and Methods
4.1. Cell Culture
4.2. Transwell Studies
4.3. Forskolin Treatment
4.4. RNA Isolation, cDNA Synthesis and Quantitative PCR
4.5. Protein Extraction and Immunoblotting
4.6. Analysis of Mercury
4.7. Statistics and Software
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gundacker, C.; Hengstschläger, M. The role of the placenta in fetal exposure to heavy metals. Wien. Med. Wochenschr. 2012, 162, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Laine, J.E.; Ray, P.; Bodnar, W.; Cable, P.H.; Boggess, K.; Offenbacher, S.; Fry, R.C. Placental cadmium levels are associated with increased preeclampsia risk. PLoS ONE 2015, 10, e0139341. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Fraser, M.; Vaillancourt, C. Inhibition of placental 11beta-hydroxysteroid dehydrogenase type 2 by lead. Reprod. Toxicol. 2016, 65, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Murcia, M.; Ballester, F.; Enning, A.M.; Iñiguez, C.; Valvi, D.; Basterrechea, M.; Rebagliato, M.; Vioque, J.; Maruri, M.; Tardon, A.; et al. Prenatal mercury exposure and birth outcomes. Environ. Res. 2016, 151, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Gundacker, C.; Fröhlich, S.; Graf-Rohrmeister, K.; Eibenberger, B.; Jessenig, V.; Gicic, D.; Prinz, S.; Wittmann, K.J.; Zeisler, H.; Vallant, B.; et al. Perinatal lead and mercury exposure in Austria. Sci. Total Environ. 2010, 408, 5744–5749. [Google Scholar] [CrossRef] [PubMed]
- Ask, K.; Akesson, A.; Berglund, M.; Vahter, M. Inorganic mercury and methylmercury in placentas of swedish women. Environ. Health Perspect. 2002, 110, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Stern, A.H.; Smith, A.E. An assessment of the cord blood: Maternal blood methylmercury ratio: Implications for risk assessment. Environ. Health Perspect. 2003, 111, 1465–1470. [Google Scholar] [CrossRef] [PubMed]
- Straka, E.; Ellinger, I.; Balthasar, C.; Scheinast, M.; Schatz, J.; Szattler, T.; Bleichert, S.; Saleh, L.; Knöfler, M.; Zeisler, H.; et al. Mercury toxicokinetics of the healthy human term placenta involve amino acid transporters and ABC transporters. Toxicology 2016, 340, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W. The three modern faces of mercury. Environ. Health Perspect. 2002, 110, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeyer, R.E.; Singh, S.P.; Doonan, C.J.; Ross, A.R.S.; Hughes, R.J.; Pickering, I.J.; George, G.N. Molecular mimicry in mercury toxicology. Chem. Res. Toxicol. 2006, 19, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Jansson, T. Amino acid transporters in the human placenta. Pediatr. Res. 2001, 49, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Verrey, F. System L: Heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch. 2003, 445, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Kerper, L.E.; Ballatori, N.; Clarkson, T.W. Methylmercury transport across the blood-brain barrier by an amino acid carrier. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1992, 262, R761–R765. [Google Scholar]
- Simmons-Willis, T.A.; Koh, A.S.; Clarkson, T.W.; Ballatori, N. Transport of a neurotoxicant by molecular mimicry: The methylmercury-l-cysteine complex is a substrate for human l-type large neutral amino acid transporter (lat) 1 and lat2. Biochem. J. 2002, 367, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Jiang, H.; Syversen, T.; Rocha, J.B.; Farina, M.; Aschner, M. The methylmercury-l-cysteine conjugate is a substrate for the l-type large neutral amino acid transporter. J. Neurochem. 2008, 107, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Heggland, I.; Kaur, P.; Syversen, T. Uptake and efflux of methylmercury in vitro: Comparison of transport mechanisms in C6, B35 and RBE4 cells. Toxicol. In Vitro 2009, 23, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, Y.; Yasutake, A.; Adachi, T.; Hirayama, K. Methylmercury transport across the placenta via neutral amino acid carrier. Arch Toxicol. 1996, 70, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Prouillac, C.; Lecoeur, S. The role of the placenta in fetal exposure to xenobiotics: Importance of membrane transporters and human models for transfer studies. Drug Metab. Dispos. 2010, 38, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, I.; Schwab, M.; Stefanescu, A.; Hunziker, W.; Fuchs, R. IgG transport across trophoblast-derived BeWo cells: A model system to study IgG transport in the placenta. Eur. J. Immunol. 1999, 29, 733–744. [Google Scholar] [CrossRef]
- Ellinger, I.; Chatuphonprasert, W.; Reiter, M.; Voss, A.; Kemper, J.; Straka, E.; Scheinast, M.; Zeisler, H.; Salzer, H.; Gundacker, C. Don’t trust an(t)ybody—Pitfalls during investigation of candidate proteins for methylmercury transport at the placental interface. Placenta 2016, 43, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.M.; Brooks, S.; Crocker, I.P.; Glazier, J.; Hanson, M.A.; Johnstone, E.D.; Panitchob, N.; Please, C.P.; Sibley, C.P.; Widdows, K.L.; et al. Review: Modelling placental amino acid transfer—From transporters to placental function. Placenta 2013, 34, S46–S51. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Jin, H.; Rytting, E.; Silverstein, P.; Young, A.; Audus, K. In vitro models for studying trophoblast transcellular transport. Methods Mol. Med. 2006, 122, 225–239. [Google Scholar] [PubMed]
- Huang, X.; Lüthi, M.; Ontsouka, E.C.; Kallol, S.; Baumann, M.U.; Surbek, D.V.; Albrecht, C. Establishment of a confluent monolayer model with human primary trophoblast cells: Novel insights into placental glucose transport. Mol. Hum. Reprod. 2016, 22, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.; Vyas, J.; Ballatori, N. Mechanisms of mercury disposition in the body. Am. J. Ind. Med. 2007, 50, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W.; Magos, L. The toxicology of mercury and its chemical compounds. Crit. Rev. Toxicol. 2006, 36, 609–662. [Google Scholar] [CrossRef] [PubMed]
- Ballatori, N. Transport of toxic metals by molecular mimicry. Environ. Health Perspect. 2002, 110, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, L.; Scalise, M.; Galluccio, M.; Pochini, L.; Albanese, L.M.; Indiveri, C. LAT1 is the transport competent unit of the LAT1/CD98 heterodimeric amino acid transporter. Int. J. Biochem. Cell Biol. 2015, 67, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Rooney, J.P.K. The role of thiols, dithiols, nutritional factors and interacting ligands in the toxicology of mercury. Toxicology 2007, 234, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Nicklin, P.; Bergman, P.; Zhang, B.; Triantafellow, E.; Wang, H.; Nyfeler, B.; Yang, H.; Hild, M.; Kung, C.; Wilson, C.; et al. Bidirectional transport of amino acids regulates mtor and autophagy. Cell 2009, 136, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Tsumura, H.; Suzuki, N.; Saito, H.; Kawano, M.; Otake, S.; Kozuka, Y.; Komada, H.; Tsurudome, M.; Ito, Y. The targeted disruption of the CD98 gene results in embryonic lethality. Biochem. Biophys. Res. Commun. 2003, 308, 847–851. [Google Scholar] [CrossRef]
- Poettler, M.; Unseld, M.; Braemswig, K.; Haitel, A.; Zielinski, C.C.; Prager, G.W. CD98hc (SLC3A2) drives integrin-dependent renal cancer cell behavior. Mol. Cancer Res. 2013, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Boulter, E.; Estrach, S.; Errante, A.; Pons, C.; Cailleteau, L.; Tissot, F.; Meneguzzi, G.; Féral, C.C. CD98hc (SLC3A2) regulation of skin homeostasis wanes with age. J. Exp. Med. 2013, 210, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Poncet, N.; Mitchell, F.E.; Ibrahim, A.F.M.; McGuire, V.A.; English, G.; Arthur, J.S.C.; Shi, Y.-B.; Taylor, P.M. The catalytic subunit of the system L1 amino acid transporter (SLC7A5) facilitates nutrient signalling in mouse skeletal muscle. PLoS ONE 2014, 9, e89547. [Google Scholar] [CrossRef] [PubMed]
- Dann, S.G.; Ryskin, M.; Barsotti, A.M.; Golas, J.; Shi, C.; Miranda, M.; Hosselet, C.; Lemon, L.; Lucas, J.; Karnoub, M.; et al. Reciprocal regulation of amino acid import and epigenetic state through Lat1 and EZH2. EMBO J. 2015, 34, 1773–1785. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.; Wirth, E.K.; Wohlgemuth, F.; Reix, N.; Klein, M.O.; Grüters, A.; Köhrle, J.; Schweizer, U. Aminoaciduria, but normal thyroid hormone levels and signalling, in mice lacking the amino acid and thyroid hormone transporter SLC7A8. Biochem. J. 2011, 439, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.; Christian, H.C.; Redman, C.W.G.; Sargent, I.L.; Boyd, C.A.R. Differential effect of cross-linking the CD98 heavy chain on fusion and amino acid transport in the human placental trophoblast (Bewo) cell line. Biochim. Biophys. Acta 2007, 1768, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, R.; Ohmori, T.; Hara, S.; Nakagomi, S.; Kanai-Azuma, M.; Kaneda-Nakashima, K.; Okuda, S.; Nagamori, S.; Kanai, Y. Essential roles of l-type amino acid transporter 1 in syncytiotrophoblast development by presenting fusogenic 4f2hc. Mol. Cell. Biol. 2017, 37, e00427-16. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.T.; Santos, D.B.; Naime, A.A.; Leal, R.B.; Dórea, J.G.; Barbosa, F. Jr.; Aschner, M.; Rocha, J.B.T.; Farina, M. Comparative study on methyl- and ethylmercury-induced toxicity in C6 glioma cells and the potential role of LAT-1 in mediating mercurial-thiol complexes uptake. Neurotoxicology 2013, 38, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Chen, C.S. Deconstructing the third dimension—How 3D culture microenvironments alter cellular cues. J. Cell Sci. 2012, 125, 3015–3024. [Google Scholar] [CrossRef] [PubMed]
- Gaccioli, F.; Aye, I.L.M.H.; Roos, S.; Lager, S.; Ramirez, V.I.; Kanai, Y.; Powell, T.L.; Jansson, T. Expression and functional characterisation of system l amino acid transporters in the human term placenta. Reprod. Biol. Endocrinol. 2015, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Ayuk, P.T.Y.; Sibley, C.P.; Donnai, P.; D’Souza, S.; Glazier, J.D. Development and polarization of cationic amino acid transporters and regulators in the human placenta. Am. J. Physiol. Cell Physiol. 2000, 278, C1162–C1171. [Google Scholar] [PubMed]
- Kudo, Y.; Boyd, C.A.R. Characterisation of l-tryptophan transporters in human placenta: A comparison of brush border and basal membrane vesicles. J. Physiol. 2001, 531, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Sakata, M.; Ogura, K.; Yamamoto, T.; Yamaguchi, M.; Tasaka, K.; Kurachi, H.; Tsurudome, M.; Murata, Y. Expression and regulation of 4f2hc and hlat1 in human trophoblasts. Am. J. Physiol. Cell Physiol. 2002, 282, C196–C204. [Google Scholar] [PubMed]
- Rosner, M.; Siegel, N.; Fuchs, C.; Slabina, N.; Dolznig, H.; Hengstschläger, M. Efficient siRNA-mediated prolonged gene silencing in human amniotic fluid stem cells. Nat. Protocols 2010, 5, 1081–1095. [Google Scholar] [CrossRef] [PubMed]




| Product Name | Company, Article No. (Dilution) |
|---|---|
| anti-4F2hc rabbit polyclonal antibody | Cell Signaling, #13180 (1:1000) |
| anti-LAT1 rabbit polyclonal antibody | Cell Signaling, #5347 (1:1000) |
| anti-LAT2 mouse monoclonal antibody | OriGene, TA500513S (1:500) |
| anti-LAT2 rabbit polyclonal antibody | Santa Cruz, sc-133726 (1:100) |
| anti-LAT2 rabbit polyclonal antibody | ImmunoGlobe, 0142-10 (1:1000) |
| anti-α-Tubulin mouse monoclonal antibody | Merck, 05-829 (1:5000) |
| mouse IgG-heavy and light chain antibody | Bethyl, A90-116P (1:10,000) |
| rabbit IgG-heavy and light chain antibody | Bethyl, A120-101P (1:10,000) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balthasar, C.; Stangl, H.; Widhalm, R.; Granitzer, S.; Hengstschläger, M.; Gundacker, C. Methylmercury Uptake into BeWo Cells Depends on LAT2-4F2hc, a System L Amino Acid Transporter. Int. J. Mol. Sci. 2017, 18, 1730. https://doi.org/10.3390/ijms18081730
Balthasar C, Stangl H, Widhalm R, Granitzer S, Hengstschläger M, Gundacker C. Methylmercury Uptake into BeWo Cells Depends on LAT2-4F2hc, a System L Amino Acid Transporter. International Journal of Molecular Sciences. 2017; 18(8):1730. https://doi.org/10.3390/ijms18081730
Chicago/Turabian StyleBalthasar, Christina, Herbert Stangl, Raimund Widhalm, Sebastian Granitzer, Markus Hengstschläger, and Claudia Gundacker. 2017. "Methylmercury Uptake into BeWo Cells Depends on LAT2-4F2hc, a System L Amino Acid Transporter" International Journal of Molecular Sciences 18, no. 8: 1730. https://doi.org/10.3390/ijms18081730
APA StyleBalthasar, C., Stangl, H., Widhalm, R., Granitzer, S., Hengstschläger, M., & Gundacker, C. (2017). Methylmercury Uptake into BeWo Cells Depends on LAT2-4F2hc, a System L Amino Acid Transporter. International Journal of Molecular Sciences, 18(8), 1730. https://doi.org/10.3390/ijms18081730