Dual Role of MiR-21-Mediated Signaling in HUVECs and Rat Surgical Flap under Normoxia and Hypoxia Condition
Abstract
:1. Introduction
2. Results
2.1. Indocyanine Green (ICG) and Laser Doppler Quantificate the Insufficient Vascularity of the Distal Skin Flap
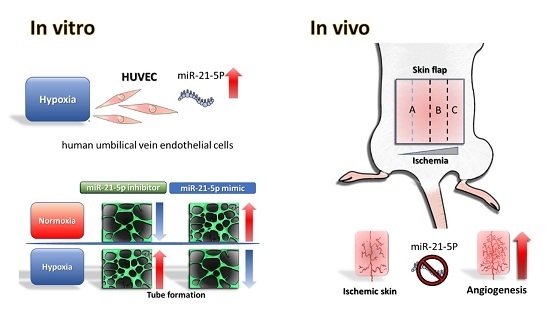
2.2. Evaluation of Hsa-MiR-21-5p Expression under Hypoxia Stress in HUVEC
2.3. Investigating the Role of Hsa-MiR-21-5p in Tube Formation Capacity in HUVEC
2.4. Investigation of Hsa-MiR-21-5p-Mediated Signaling Molecules under Hypoxia Stress in HUVEC
2.5. Investigating the Effect MiR-21-5p on Blood Flow in the Skin Flap
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Quantification of MiR-21-5p
4.3. Tube Formation Assay
4.4. Western Blotting
4.5. Reporter Assay
4.6. Rat Skin Flap Surgery
4.7. Assessment of Blood Flow in Rat Flap
4.8. Delivery of MiRNA
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, K.; Zhang, M.; Hu, J.; Liu, Y.; Wang, Y.; Ma, X. Methane-rich saline attenuates ischemia/reperfusion injury of abdominal skin flaps in rats via regulating apoptosis level. BMC Surg. 2015, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Kriegel, A.J.; Jiao, X.; Liu, H.; Bai, X.; Olson, J.; Liang, M.; Ding, X. Mir-21 in ischemia/reperfusion injury: A double-edged sword? Physiol. Genom. 2014, 46, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Harder, Y.; Amon, M.; Laschke, M.W.; Schramm, R.; Rucker, M.; Wettstein, R.; Bastiaanse, J.; Frick, A.; Machens, H.G.; Kuntscher, M.; et al. An old dream revitalised: Preconditioning strategies to protect surgical flaps from critical ischaemia and ischaemia-reperfusion injury. J. Plast. Reconstr. Aesthet. Surg. 2008, 61, 503–511. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a big role in gene regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Jeyaseelan, K.; Lim, K.Y.; Armugam, A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 2008, 39, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.P.; Wu, J.; Wang, X.; Sartor, M.A.; Qian, J.; Jones, K.; Nicolaou, P.; Pritchard, T.J.; Fan, G.C. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation 2009, 119, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Godwin, J.G.; Ge, X.; Stephan, K.; Jurisch, A.; Tullius, S.G.; Iacomini, J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc. Natl. Acad. Sci. USA 2010, 107, 14339–14344. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.; Meng, Y.; Xie, Q.; Yi, W.J.; Lai, X.L.; Bian, Q.; Wang, J.; Wang, J.F.; Yu, G. Mir-98 protects endothelial cells against hypoxia/reoxygenation induced-apoptosis by targeting caspase-3. Biochem. Biophys. Res. Commun. 2015, 467, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.P.; Lai, C.S. MicroRNA profiling as biomarkers in flap ischemia-reperfusion injury. Microsurgery 2012, 32, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Khouri, R.K. Avoiding free flap failure. Clin. Plast. Surg. 1992, 19, 773–781. [Google Scholar] [PubMed]
- Pang, C.Y.; Forrest, C.R.; Morris, S.F. Pharmacological augmentation of skin flap viability: A hypothesis to mimic the surgical delay phenomenon or a wishful thought. Ann. Plast. Surg. 1989, 22, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fan, G.C. Role of microRNAs in the reperfused myocardium towards post-infarct remodelling. Cardiovasc. Res. 2012, 94, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Cao, X.; Zou, L.; Chen, Y.; Guo, J.; Chen, Z.; Hu, S.; Zheng, Z. MicroRNA-21 and risk of severe acute kidney injury and poor outcomes after adult cardiac surgery. PLoS ONE 2013, 8, e63390. [Google Scholar] [CrossRef] [PubMed]
- Rink, C.; Khanna, S. MicroRNA in ischemic stroke etiology and pathology. Physiol. Genom. 2011, 43, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Banerjee, S.; Freitas, A.; Cui, H.; Xie, N.; Abraham, E.; Liu, G. Mir-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L521–L529. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Song, N.; Zhang, X.; Jiao, X.; Hu, J.; Liang, M.; Teng, J.; Ding, X. Renal protection mediated by hypoxia inducible factor-1α depends on pro-angiogenesis function of mir-21 by targeting thrombospondin 1. Transplantation 2017, 101, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, N. Mir-21 might be involved in breast cancer promotion and invasion rather than in initial events of breast cancer development. Mol. Diagn. Ther. 2016, 20, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Y.; Zhao, G. Emerging role of microRNA-21 in colorectal cancer. Cancer Biomark. 2015, 15, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Hermansen, S.K.; Nielsen, B.S.; Aaberg-Jessen, C.; Kristensen, B.W. Mir-21 is linked to glioma angiogenesis: A co-localization study. J. Histochem. Cytochem. 2016, 64, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Guduric-Fuchs, J.; O’Connor, A.; Cullen, A.; Harwood, L.; Medina, R.J.; O’Neill, C.L.; Stitt, A.W.; Curtis, T.M.; Simpson, D.A. Deep sequencing reveals predominant expression of mir-21 amongst the small non-coding RNAs in retinal microvascular endothelial cells. J. Cell. Biochem. 2012, 113, 2098–2111. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.S.; Tian, S.S.; Lu, J.J.; Ding, X.H.; Qian, C.D.; Ding, B.; Ding, Z.S.; Jin, B. Cardamonin regulates mir-21 expression and suppresses angiogenesis induced by vascular endothelial growth factor. Biomed Res. Int. 2015, 2015, 501581. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- Krek, A.; Grun, D.; Poy, M.N.; Wolf, R.; Rosenberg, L.; Epstein, E.J.; MacMenamin, P.; da Piedade, I.; Gunsalus, K.C.; Stoffel, M.; et al. Combinatorial microRNA target predictions. Nat. Genet. 2005, 37, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Betel, D.; Wilson, M.; Gabow, A.; Marks, D.S.; Sander, C. The microRNA.Org resource: Targets and expression. Nucleic Acids Res. 2008, 36, D149–D153. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.; Wang, X. Mirdb: An online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2015, 43, D146–D152. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, D.; Wang, Y.; Sun, P.; Hou, X.; Larner, J.; Xiong, W.; Mi, J. Mir-21/SMAD 7 signaling determines TGF-β1-induced caf formation. Sci. Rep. 2013, 3, 2038. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Tan, X.; Mu, L.; Luo, Y.; Li, R.; Deng, X.; Chen, N.; Ren, M.; Li, Y.; Wang, L.; et al. MiRNA-21 mediates the antiangiogenic activity of metformin through targeting PTEN and SMAD7 expression and PI3K/AKT pathway. Sci. Rep. 2017, 7, 43427. [Google Scholar] [CrossRef] [PubMed]
- McClelland, A.D.; Herman-Edelstein, M.; Komers, R.; Jha, J.C.; Winbanks, C.E.; Hagiwara, S.; Gregorevic, P.; Kantharidis, P.; Cooper, M.E. Mir-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin. Sci. 2015, 129, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Nie, Y.; Qu, S.; Liao, J.Y.; Cui, X.; Yao, H.; Zeng, Y.; Su, F.; Song, E.; Liu, Q. Mir-21 induces myofibroblast differentiation and promotes the malignant progression of breast phyllodes tumors. Cancer Res. 2014, 74, 4341–4352. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Zhao, Y.; Yu, L.; Xu, S.; Fu, G. MicroRNA-21 mediates the rapamycin-induced suppression of endothelial proliferation and migration. FEBS Lett. 2013, 587, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Suarez, Y.; Fernandez-Hernando, C.; Pober, J.S.; Sessa, W.C. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ. Res. 2007, 100, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, A.; Li, T.; Su, X.; Ding, H.; Li, H.; Qin, X.; Hou, L.; Zhao, Q.; Ge, X.; et al. Mir-152 reduces human umbilical vein endothelial cell proliferation and migration by targeting ADAM17. FEBS. Lett. 2014, 588, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Madanecki, P.; Kapoor, N.; Bebok, Z.; Ochocka, R.; Collawn, J.F.; Bartoszewski, R. Regulation of angiogenesis by hypoxia: The role of microRNA. Cell. Mol. Biol. Lett. 2013, 18, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Ergul, A.; Alhusban, A.; Fagan, S.C. Angiogenesis: A harmonized target for recovery after stroke. Stroke 2012, 43, 2270–2274. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.P.; He, S.Y.; Xu, B.; Hu, C.J.; Lu, S.F.; Shen, W.X.; Huang, Y.; Hong, H.; Li, Q.; Wang, N.; et al. Acupuncture promotes angiogenesis after myocardial ischemia through H3K9 acetylation regulation at VEGF gene. PLoS ONE 2014, 9, e94604. [Google Scholar] [CrossRef] [PubMed]
- Iruela-Arispe, L.; Gordon, K.; Hugo, C.; Duijvestijn, A.M.; Claffey, K.P.; Reilly, M.; Couser, W.G.; Alpers, C.E.; Johnson, R.J. Participation of glomerular endothelial cells in the capillary repair of glomerulonephritis. Am. J. Pathol. 1995, 147, 1715–1727. [Google Scholar] [PubMed]
- Kuehbacher, A.; Urbich, C.; Zeiher, A.M.; Dimmeler, S. Role of dicer and drosha for endothelial microRNA expression and angiogenesis. Circ. Res. 2007, 101, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Dou, X.; Hao, W.; Zhou, Q.; Tang, R.; Nie, J.; Lan, H.Y.; Yu, X. SMAD7 gene transfer attenuates angiogenesis in peritoneal dialysis rats. Nephrology 2013, 18, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Teng, J.; Zou, J.; Fang, Y.; Zhang, X.; Bosnjak, Z.J.; Liang, M.; Ding, X. Mir-21 contributes to xenon-conferred amelioration of renal ischemia-reperfusion injury in mice. Anesthesiology 2013, 119, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, Y.; Zhang, L.; Wu, T.; Wu, T.; Zhang, W.; Decker, A.M.; He, J.; Liu, J.; Wu, Y.; et al. Human stem cells overexpressing mir-21 promote angiogenesis in critical limb ischemia by targeting chip to enhance hif-1α activity. Stem Cells 2016, 34, 924–934. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Z.; Li, C.; Chen, Q.; Jing, Y.; Carpenter, R.; Jiang, Y.; Kung, H.F.; Lai, L.; Jiang, B.H. Mir-21 induced angiogenesis through AKT and ERK activation and hif-1α expression. PLoS ONE 2011, 6, e19139. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Gao, Y.B.; Zhang, N.; Zou, D.W.; Wang, P.; Zhu, Z.Y.; Li, J.Y.; Zhou, S.N.; Wang, S.C.; Wang, Y.Y.; et al. Mir-21 overexpression enhances TGF-β1-induced epithelial-to-mesenchymal transition by target SMAD7 and aggravates renal damage in diabetic nephropathy. Mol. Cell. Endocrinol. 2014, 392, 163–172. [Google Scholar] [CrossRef] [PubMed]






© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-H.; Yen, M.-C.; Liao, S.-H.; Hsu, Y.-L.; Lai, C.-S.; Kuo, Y.-R.; Hsu, Y.-L. Dual Role of MiR-21-Mediated Signaling in HUVECs and Rat Surgical Flap under Normoxia and Hypoxia Condition. Int. J. Mol. Sci. 2017, 18, 1917. https://doi.org/10.3390/ijms18091917
Chang C-H, Yen M-C, Liao S-H, Hsu Y-L, Lai C-S, Kuo Y-R, Hsu Y-L. Dual Role of MiR-21-Mediated Signaling in HUVECs and Rat Surgical Flap under Normoxia and Hypoxia Condition. International Journal of Molecular Sciences. 2017; 18(9):1917. https://doi.org/10.3390/ijms18091917
Chicago/Turabian StyleChang, Chih-Hau, Meng-Chi Yen, Ssu-Hui Liao, Yu-Ling Hsu, Chung-Sheng Lai, Yur-Ren Kuo, and Ya-Ling Hsu. 2017. "Dual Role of MiR-21-Mediated Signaling in HUVECs and Rat Surgical Flap under Normoxia and Hypoxia Condition" International Journal of Molecular Sciences 18, no. 9: 1917. https://doi.org/10.3390/ijms18091917
APA StyleChang, C. -H., Yen, M. -C., Liao, S. -H., Hsu, Y. -L., Lai, C. -S., Kuo, Y. -R., & Hsu, Y. -L. (2017). Dual Role of MiR-21-Mediated Signaling in HUVECs and Rat Surgical Flap under Normoxia and Hypoxia Condition. International Journal of Molecular Sciences, 18(9), 1917. https://doi.org/10.3390/ijms18091917