Introduction of Exogenous HSV-TK Suicide Gene Increases Safety of Keratinocyte-Derived Induced Pluripotent Stem Cells by Providing Genetic “Emergency Exit” Switch
Abstract
:1. Introduction
2. Results
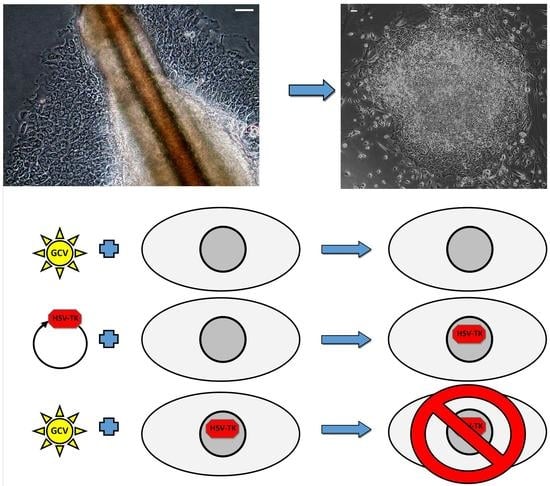
2.1. Reprogramming of Keratinocytes from Plucked Hair
2.2. Characteristic of Acquired kiPS Cell Lines
2.3. Genetic Modification of kiPS Cell Lines
2.4. Successful Suicide Gene Therapy of kiPS Cells In Vitro an In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Isolation of Keratinocytes from Plucked Hair
4.3. Keratinocytes Reprogramming into iPS Cells
4.4. Immunocytochemistry
4.5. RT-PCR
4.6. Teratoma Formation
4.7. Generation and Analysis of Genetically Modified Cells
4.8. Suicide Gene Therapy In Vitro
4.9. Xenografts and Suicide Gene Therapy In Vivo
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| iPS | induced Pluripotent Stem cells |
| kiPS | eratinocytes-derived-iPS |
| piPS | protein-iPS |
| HSV-TK | Herpes Simplex Virus Thimidine Kinase |
| GCV | Ganciclovir |
| GFP | Green Fluorescence Protein |
References
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K. Direct reprogramming 101. Dev. Growth Differ. 2010, 52, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Egawa, N.; Kitaoka, S.; Tsukita, K.; Naitoh, M.; Takahashi, K.; Yamamoto, T.; Adachi, F.; Kondo, T.; Okita, K.; Asaka, I.; et al. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci. Transl. Med. 2012, 4, 145ra104. [Google Scholar] [CrossRef] [PubMed]
- Sirenko, O.; Cromwell, E.F.; Crittenden, C.; Wignall, J.A.; Wright, F.A.; Rusyn, I. Assessment of beating parameters in human induced pluripotent stem cells enables quantitative in vitro screening for cardiotoxicity. Toxicol. Appl. Pharmacol. 2013, 273, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Dimos, J.T.; Rodolfa, K.T.; Niakan, K.K.; Weisenthal, L.M.; Mitsumoto, H.; Chung, W.; Croft, G.F.; Saphier, G.; Leibel, R.; Goland, R.; et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 2008, 321, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Vergara, X.; Sevilla, A.; D’Souza, S.L.; Ang, Y.-S.; Schaniel, C.; Lee, D.-F.; Yang, L.; Kaplan, A.D.; Adler, E.D.; Rozov, R.; et al. Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome. Nature 2010, 465, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-H.; Arora, N.; Huo, H.; Maherali, N.; Ahfeldt, T.; Shimamura, A.; Lensch, M.W.; Cowan, C.; Hochedlinger, K.; Daley, G.Q. Disease-specific induced pluripotent stem cells. Cell 2008, 134, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Soldner, F.; Hockemeyer, D.; Beard, C.; Gao, Q.; Bell, G.W.; Cook, E.G.; Hargus, G.; Blak, A.; Cooper, O.; Mitalipova, M.; et al. Parkinson’s Disease Patient-Derived Induced Pluripotent Stem Cells Free of Viral Reprogramming Factors. Cell 2009, 136, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Danés, A.; Richaud-Patin, Y.; Carballo-Carbajal, I.; Jiménez-Delgado, S.; Caig, C.; Mora, S.; Di Guglielmo, C.; Ezquerra, M.; Patel, B.; Giralt, A.; et al. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol. Med. 2012, 4, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Kaifeng, S.; Dittmann, S.; Xu, G.; Gupta, M.K.; Linke, M.; Zechner, U.; Nguemo, F.; Milting, H.; Farr, M.; et al. The Disease-Specific Phenotype in Cardiomyocytes Derived from Induced Pluripotent Stem Cells of Two Long QT Syndrome Type 3 Patients. PLoS ONE 2013, 8, e83005. [Google Scholar] [CrossRef] [PubMed]
- Ebert, A.D.; Yu, J.; Rose, F.F.; Mattis, V.B.; Lorson, C.L.; Thomson, J.A.; Svendsen, C.N. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 2009, 457, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, C.; Moon, J.; Chung, Y.; Chang, M.; Han, B.; Ko, S.; Yang, E.; Cha, K.Y.; Lanza, R.; et al. Generation of Human Induced Pluripotent Stem Cells by Direct Delivery of Reprogramming Proteins. Cell Stem Cell 2009, 4, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wu, S.; Joo, J.Y.; Zhu, S.; Han, D.W.; Lin, T.; Trauger, S.; Bien, G.; Yao, S.; Zhu, Y.; et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 2009, 4, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Yusa, K.; Rad, R.; Takeda, J.; Bradley, A. Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nature 2009, 6, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Woltjen, K.; Michael, I.P.; Mohseni, P.; Desai, R.; Mileikovsky, M.; Hämäläinen, R.; Cowling, R.; Wang, W.; Liu, P.; Gertsenstein, M.; et al. PiggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 2009, 458, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.; Manos, P.D.; Ahfeldt, T.; Loh, Y.-H.; Li, H.; Lau, F.; Ebina, W.; Mandal, P.K.; Smith, Z.D.; Meissner, A.; et al. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010, 7, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Nakagawa, M.; Hyenjong, H.; Ichisaka, T.; Yamanaka, S. Generation of mouse induced pluripotent stem cells without viral vectors. Science 2008, 322, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Fusaki, N.; Ban, H.; Nishiyama, A.; Saeki, K.; Hasegawa, M. Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc. Jpn. Acad. Ser. B 2009, 85, 348–362. [Google Scholar] [CrossRef]
- Hou, P.; Li, Y.; Zhang, X.; Liu, C.; Guan, J.; Li, H.; Zhao, T.; Ye, J.; Yang, W.; Liu, K.; et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science 2013, 341, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Masuda, S.; Wu, J.; Hishida, T.; Pandian, G.N.; Sugiyama, H.; Izpisua Belmonte, J.C. Chemically induced pluripotent stem cells (CiPSCs): A transgene-free approach. J. Mol. Cell Biol. 2013, 5, 354–355. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Belmonte, J.C.I. No factor left behind: Generation of transgene-free in-duced pluripotent stem cells. Am. J. Stem Cells 2012, 1, 75–80. [Google Scholar] [PubMed]
- Zhao, T.; Zhang, Z.-N.; Rong, Z.; Xu, Y. Immunogenicity of induced pluripotent stem cells. Nature 2011, 474, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Chen, M.; Yang, B.; Zhang, F.; Cao, K. Intramyocardial transplantation of undifferentiated rat induced pluripotent stem cells causes tumorigenesis in the heart. PLoS ONE 2011, 6, e19012. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Yamashita, T.; Ohta, Y.; Deguchi, K.; Nagotani, S.; Zhang, X.; Ikeda, Y.; Matsuura, T.; Abe, K. Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. J. Cereb. Blood Flow Metab. 2010, 30, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Zeng, Q.; Fan, Z.; Li, J.; Zhang, M.; Zhang, Y.; Li, O.; Chen, L.; Kong, X.; Zhang, H. Monitoring HSV-TK/ganciclovir cancer suicide gene therapy using CdTe/CdS core/shell quantum dots. Biomaterials 2012, 33, 4336–4344. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Cayeux, S.; Lupton, S.D.; Dorken, B.; Blankenstees, T. The Thymidine Kinase/Ganciclovir-Mediated “Suicide” Effect Is Variable in Different Tumor Cells. Hum. Gene Ther. 1995, 6, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, P.; Sułkowski, M.; Badyra, B.; Kijowski, J.; Majka, M. Suicide gene therapy of rhabdomyosarcoma. Int. J. Oncol. 2017, 50, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.; Panis, Y.; Gagandeep, S.; Houssin, D.; Salzmann, J.L.; Klatzmann, D. Regression of established macroscopic liver metastases after in situ transduction of a suicide gene. Proc. Natl. Acad. Sci. USA 1993, 90, 7024–7028. [Google Scholar] [CrossRef] [PubMed]
- Wildner, O.; Morris, J.C.; Vahanian, N.N.; Ford, H.; Ramsey, W.J.; Blaese, R.M. Adenoviral vectors capable of replication improve the efficacy of HSVtk/GCV suicide gene therapy of cancer. Gene Ther. 1999, 6, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.D.; Gomez-Navarro, J.; Wang, M.; Barnes, M.N.; Strong, T.V.; Arani, R.B.; Arafat, W.; Hughes, J.V.; Siegal, G.P.; Curiel, D.T. Adenoviral-mediated suicide gene therapy for ovarian cancer. Mol. Ther. 2000, 2, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Kokoris, M.S.; Black, M.E. Characterization of herpes simplex virus type 1 thymidine kinase mutants engineered for improved ganciclovir or acyclovir activity. Protein Sci. 2002, 11, 2267–2272. [Google Scholar] [CrossRef] [PubMed]
- Tomicic, M.T.; Thust, R.; Kaina, B. Ganciclovir-induced apoptosis in HSV-1 thymidine kinase expressing cells: Critical role of DNA breaks, Bcl-2 decline and caspase-9 activation. Oncogene 2002, 21, 2141–2153. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, T.W.; Read, S.B.; Burrows, F.J.; Kruse, C.A. Suicide gene therapy with Herpes simplex virus thymidine kinase and ganciclovir is enhanced with connexins to improve gap junctions and bystander effects. Histol. Histopathol. 2003, 18, 495–507. [Google Scholar] [PubMed]
- Czyż, J.; Piwowarczyk, K.; Paw, M.; Luty, M.; Wróbel, T.; Catapano, J.; Madeja, Z.; Ryszawy, D. Connexin-dependent intercellular stress signaling in tissue homeostasis and tumor development. Acta Biochim. Pol. 2017, 64, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Rozental, A.; Rozental, R.; Hopperstad, M.; Wu, J.; Vrionis, F.; Spray, D. Gap junctions: The “kiss of death” and the “kiss of life”. Brain Res. Rev. 2000, 32, 308–315. [Google Scholar] [CrossRef]
- Li, R.; Liang, J.; Ni, S.; Zhou, T.; Qing, X.; Li, H.; He, W.; Chen, J.; Li, F.; Zhuang, Q.; et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell 2010, 7, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Aasen, T.; Raya, A.; Barrero, M.J.; Garreta, E.; Consiglio, A.; Gonzalez, F.; Vassena, R.; Bilić, J.; Pekarik, V.; Tiscornia, G.; et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol. 2008, 26, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, Y.; Asakawa, M.; Kuma, H.; Hirata, T.; Ueda, Y.; Lee, Y.; Iida, A.; Kato, A.; Nagai, Y.; et al. A Cytoplasmic RNA Vector Derived from Nontransmissible Sendai virus with Efficient Gene Transfer and Expression. J. Virol. 2000, 74, 6564–6569. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Sano, M.; Ohtaka, M.; Furuta, B.; Umemura, Y.; Nakajima, Y.; Ikehara, Y.; Kobayashi, T.; Segawa, H.; Takayasu, S.; et al. Development of defective and persistent Sendai virus vector: A unique gene delivery/expression system ideal for cell reprogramming. J. Biol. Chem. 2011, 286, 4760–4771. [Google Scholar] [CrossRef] [PubMed]
- Moll, I. Proliferative potential of different keratinocytes of plucked human hair follicles. J. Investig. Dermatol. 1995, 105, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Maherali, N.; Hochedlinger, K. Guidelines and Techniques for the Generation of Induced Pluripotent Stem Cells. Cell Stem Cell 2008, 3, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Mátrai, J.; Chuah, M.K.L.; VandenDriessche, T. Recent advances in lentiviral vector development and applications. Mol. Ther. 2010, 18, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Kay, M.A.; Glorioso, J.C.; Naldini, L. Viral vectors for gene therapy: The art of turning infectious agents into vehicles of therapeutics. Nat. Med. 2001, 7, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Mizuguchi, H.; Xu, Z.; Ishii-Watabe, A.; Uchida, E.; Hayakawa, T. IRES-Dependent Second Gene Expression Is Significantly Lower Than Cap-Dependent First Gene Expression in a Bicistronic Vector. Mol. Ther. 2000, 1, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Teschendorf, C.; Warrington, K.H.; Siemann, D.W.; Muzyczka, N. Comparison of the EF-1 alpha and the CMV promoter for engineering stable tumor cell lines using recombinant adeno-associated virus. Anticancer Res. 2002, 22, 3325–3330. [Google Scholar] [PubMed]
- Neschadim, A.; Wang, J.C.M.; Lavie, A.; Medin, J.A. Bystander killing of malignant cells via the delivery of engineered thymidine-active deoxycytidine kinase for suicide gene therapy of cancer. Cancer Gene Ther. 2012, 19, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Watts, K.L.; Gori, J.L.; Wohlfahrt, M.E.; Enssle, J.; Adair, J.E.; Kiem, H.-P. Safeguarding nonhuman primate iPS cells with suicide genes. Mol. Ther. 2011, 19, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Schuldner, M.; Itskovitz-Eldor, J.; Benvenisty, N. Selective Ablation of Human Embryonic Stem Cells Expressing a “Suicide” Gene. Stem Cells 2003, 21, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Veldwijk, M.R.; Berlinghoff, S.; Laufs, S.; Hengge, U.R.; Zeller, W.J.; Wenz, F.; Fruehauf, S. Suicide gene therapy of sarcoma cell lines using recombinant adeno-associated virus 2 vectors. Cancer Gene Ther. 2004, 11, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Freytag, S.O.; Khil, M.; Stricker, H.; Peabody, J.; Menon, M.; Deperalta-venturina, M.; Nafziger, D.; Pegg, J.; Paielli, D.; Brown, S.; et al. Phase I Study of Replication-competent Adenovirus-mediated Double Suicide Gene Therapy for the Treatment of Locally Recurrent Prostate Cancer. Cancer Res. 2002, 62, 4968–4976. [Google Scholar] [PubMed]
- Aasen, T.; Belmonte, J.C.I. Isolation and cultivation of human keratinocytes from skin or plucked hair for the generation of induced pluripotent stem cells. Nat. Protoc. 2010, 5, 371–382. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sułkowski, M.; Konieczny, P.; Chlebanowska, P.; Majka, M. Introduction of Exogenous HSV-TK Suicide Gene Increases Safety of Keratinocyte-Derived Induced Pluripotent Stem Cells by Providing Genetic “Emergency Exit” Switch. Int. J. Mol. Sci. 2018, 19, 197. https://doi.org/10.3390/ijms19010197
Sułkowski M, Konieczny P, Chlebanowska P, Majka M. Introduction of Exogenous HSV-TK Suicide Gene Increases Safety of Keratinocyte-Derived Induced Pluripotent Stem Cells by Providing Genetic “Emergency Exit” Switch. International Journal of Molecular Sciences. 2018; 19(1):197. https://doi.org/10.3390/ijms19010197
Chicago/Turabian StyleSułkowski, Maciej, Paweł Konieczny, Paula Chlebanowska, and Marcin Majka. 2018. "Introduction of Exogenous HSV-TK Suicide Gene Increases Safety of Keratinocyte-Derived Induced Pluripotent Stem Cells by Providing Genetic “Emergency Exit” Switch" International Journal of Molecular Sciences 19, no. 1: 197. https://doi.org/10.3390/ijms19010197
APA StyleSułkowski, M., Konieczny, P., Chlebanowska, P., & Majka, M. (2018). Introduction of Exogenous HSV-TK Suicide Gene Increases Safety of Keratinocyte-Derived Induced Pluripotent Stem Cells by Providing Genetic “Emergency Exit” Switch. International Journal of Molecular Sciences, 19(1), 197. https://doi.org/10.3390/ijms19010197