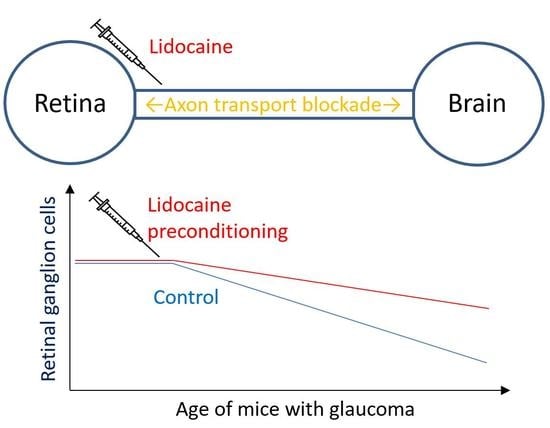
Anesthetic Preconditioning as Endogenous Neuroprotection in Glaucoma
Abstract
:1. Introduction
2. Results
2.1. Retrobulbar Lidocaine Impairs Axon Transport
2.2. Retrobulbar Lidocaine Reversibly Impairs RGC Function
2.3. Lidocaine Treatment Does not Induce Long-Term Changes of IOP and RGC Function
2.4. Short-Term Lidocaine Treatment Induces Long-Term Changes of Protein Expression in DBA/2J Glaucoma
2.5. Short-Term Lidocaine Treatment Results in Improved Long-Term Survival of Functional RGCs
3. Discussion
4. Materials and Methods
4.1. Animals and Husbandry
4.2. Pattern Electroretinogram (PERG) Recording
4.3. Western Blots
4.4. RGC Density
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef] [PubMed]
- Porciatti, V.; Ventura, L.M. Retinal ganglion cell functional plasticity and optic neuropathy: A comprehensive model. J. Neuroophthalmol. 2012, 32, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Jampel, H.D.; Chon, B.H.; Stamper, R.; Packer, M.; Han, Y.; Nguyen, Q.H.; Ianchulev, T. Effectiveness of intraocular pressure-lowering medication determined by washout. JAMA Ophthalmol. 2014, 132, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.A.; Crowe, M.E.; Quigley, H.A. Neuroprotection for glaucoma: Requirements for clinical translation. Exp. Eye Res. 2017, 157, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Leng, T.; Gao, X.; Dilger, J.P.; Lin, J. Neuroprotective effect of lidocaine: Is there clinical potential? Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 9–13. [Google Scholar] [PubMed]
- Cummins, T.R. Setting up for the block: The mechanism underlying lidocaine’s use-dependent inhibition of sodium channels. J. Physiol. 2007, 582, 11. [Google Scholar] [CrossRef] [PubMed]
- Fagiolini, M.; Caleo, M.; Strettoi, E.; Maffei, L. Axonal transport blockade in the neonatal rat optic nerve induces limited retinal ganglion cell death. J. Neurosci. 1997, 17, 7045–7052. [Google Scholar] [PubMed]
- Kanai, A.; Hiruma, H.; Katakura, T.; Sase, S.; Kawakami, T.; Hoka, S. Low-concentration lidocaine rapidly inhibits axonal transport in cultured mouse dorsal root ganglion neurons. Anesthesiology 2001, 95, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Inman, D.M.; Harun-Or-Rashid, M. Metabolic vulnerability in the neurodegenerative disease glaucoma. Front. Neurosci. 2017, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.H.; Park, K.K.; Luo, X.; Porciatti, V. Retrograde signaling in the optic nerve is necessary for electrical responsiveness of retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Kafitz, K.W.; Rose, C.R.; Konnerth, A. Neurotrophin-evoked rapid excitation of central neurons. Prog. Brain Res. 2000, 128, 243–249. [Google Scholar] [PubMed]
- Blum, R.; Kafitz, K.W.; Konnerth, A. Neurotrophin-evoked depolarization requires the sodium channel NaV1.9. Nature 2002, 419, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Porciatti, V. Electrophysiological assessment of retinal ganglion cell function. Exp. Eye Res. 2015, 141, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Nagaraju, M.; Porciatti, V. Longitudinal evaluation of retinal ganglion cell function and iop in the DBA/2J mouse model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4564–4572. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Harder, J.M.; Foxworth, N.E.; Cochran, K.E.; Philip, V.M.; Porciatti, V.; Smithies, O.; John, S.W. Vitamin b3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 2017, 355, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.R.; Inman, D.M.; Calkins, D.J.; Horner, P.J.; Vetter, M.L. Microarray analysis of retinal gene expression in the DBA/2J model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2006, 47, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Libby, R.T.; Anderson, M.G.; Pang, I.H.; Robinson, Z.H.; Savinova, O.V.; Cosma, I.M.; Snow, A.; Wilson, L.A.; Smith, R.S.; Clark, A.F.; et al. Inherited glaucoma in DBA/2J mice: Pertinent disease features for studying the neurodegeneration. Vis. Neurosci. 2005, 22, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Schuettauf, F.; Rejdak, R.; Walski, M.; Frontczak-Baniewicz, M.; Voelker, M.; Blatsios, G.; Shinoda, K.; Zagorski, Z.; Zrenner, E.; Grieb, P. Retinal neurodegeneration in the dba/2j mouse-a model for ocular hypertension. Acta Neuropathol. 2004, 107, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.N.; Inman, D.M.; Dengler Crish, C.M.; Smith, M.A.; Crish, S.D. Early pro-inflammatory cytokine elevations in the dba/2j mouse model of glaucoma. J. Neuroinflamm. 2015, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.M.; Braine, C.E.; Williams, P.A.; Zhu, X.; MacNicoll, K.H.; Sousa, G.L.; Buchanan, R.A.; Smith, R.S.; Libby, R.T.; Howell, G.R.; et al. Early immune responses are independent of rgc dysfunction in glaucoma with complement component c3 being protective. Proc. Natl. Acad. Sci. USA 2017, 114, e3839–e3848. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Peyman, G.A.; Sun, G. Toxicity of intraocular lidocaine and bupivacaine. Am. J. Ophthalmol. 1998, 125, 191–196. [Google Scholar] [CrossRef]
- Kimura, A.; Namekata, K.; Guo, X.; Harada, C.; Harada, T. Neuroprotection, growth factors and bdnf-trkb signalling in retinal degeneration. Int. J. Mol. Sci. 2016, 17, e1584. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; You, Y.; Gupta, V.B.; Klistorner, A.; Graham, S.L. Trkb receptor signalling: Implications in neurodegenerative, psychiatric and proliferative disorders. Int. J. Mol. Sci. 2013, 14, 10122–10142. [Google Scholar] [CrossRef] [PubMed]
- Pease, M.E.; McKinnon, S.J.; Quigley, H.A.; Kerrigan-Baumrind, L.A.; Zack, D.J. Obstructed axonal transport of bdnf and its receptor trkb in experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 2000, 41, 764–774. [Google Scholar]
- Chong, R.S.; Martin, K.R. Glial cell interactions and glaucoma. Curr. Opin. Ophthalmol. 2015, 26, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G.; Yang, X.; Luo, C.; Cai, J.; Powell, D.W. An astrocyte-specific proteomic approach to inflammatory responses in experimental rat glaucoma. Investig. Ophthalmol. Vis. Sci. 2012, 53, 4220–4233. [Google Scholar] [CrossRef] [PubMed]
- Schuettauf, F.; Quinto, K.; Naskar, R.; Zurakowski, D. Effects of anti-glaucoma medications on ganglion cell survival: The dba/2j mouse model. Vis. Res. 2002, 42, 2333–2337. [Google Scholar] [CrossRef]
- Sawada, K.; Hiraoka, M.; Ohguro, H. Effect of antiglaucoma medicine on intraocular pressure in dba/2j mice. Ophthalmic Res. 2016, 55, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Lev, R.; Rosen, P. Prophylactic lidocaine use preintubation: A review. J. Emerg. Med. 1994, 12, 499–506. [Google Scholar] [CrossRef]
- Jin, X.; Xue, A.; Zhao, Y.; Qin, Q.; Dong, X.D.; Qu, J. Efficacy and safety of intravenous injection of lidocaine in the treatment of acute primary angle-closure glaucoma: A pilot study. Graefes Arch. Clin. Exp. Ophthalmol. 2007, 245, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Li, B.; Rosenbaum, P.S.; Gupta, H.; Goldstein, I.M.; Maxwell, K.M.; Gidday, J.M. Preconditioning provides complete protection against retinal ischemic injury in rats. Investig. Ophthalmol. Vis. Sci. 1998, 39, 777–785. [Google Scholar]
- Liu, X.; Liang, J.P.; Sha, O.; Wang, S.J.; Li, H.G.; Cho, E.Y.P. Protection of retinal ganglion cells against optic nerve injury by induction of ischemic preconditioning. Int. J. Ophthalmol. 2017, 10, 854–861. [Google Scholar] [PubMed]
- Li, Y.; Roth, S.; Laser, M.; Ma, J.X.; Crosson, C.E. Retinal preconditioning and the induction of heat-shock protein 27. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1299–1304. [Google Scholar] [CrossRef]
- Yang, X.; Chou, T.H.; Ruggeri, M.; Porciatti, V. A new mouse model of inducible, chronic retinal ganglion cell dysfunction not associated with cell death. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1898–1904. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.C.; Jia, L.; Cepurna, W.O.; Doser, T.A.; Morrison, J.C. Global changes in optic nerve head gene expression after exposure to elevated intraocular pressure in a rat glaucoma model. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3161–3177. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.C.; Cepurna, W.O.; Tehrani, S.; Choe, T.E.; Jayaram, H.; Lozano, D.C.; Fortune, B.; Johnson, E.C. A period of controlled elevation of iop (CEI) produces the specific gene expression responses and focal injury pattern of experimental rat glaucoma. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6700–6711. [Google Scholar] [CrossRef] [PubMed]
- John, S.W.; Smith, R.S.; Savinova, O.V.; Hawes, N.L.; Chang, B.; Turnbull, D.; Davisson, M.; Roderick, T.H.; Heckenlively, J.R. Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Investig. Ophthalmol. Vis. Sci. 1998, 39, 951–962. [Google Scholar]
- Howell, G.R.; Libby, R.T.; Jakobs, T.C.; Smith, R.S.; Phalan, F.C.; Barter, J.W.; Barbay, J.M.; Marchant, J.K.; Mahesh, N.; Porciatti, V.; et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J. Cell Biol. 2007, 179, 1523–1537. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.H.; Bohorquez, J.; Toft-Nielsen, J.; Ozdamar, O.; Porciatti, V. Robust mouse pattern electroretinograms derived simultaneously from each eye using a common snout electrode. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, M.; Saleh, M.; Porciatti, V. Iop-dependent retinal ganglion cell dysfunction in glaucomatous DBA/2J mice. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4573–4579. [Google Scholar] [CrossRef] [PubMed]
- Remtulla, S.; Hallett, P.E. A schematic eye for the mouse, and comparisons with the rat. Vis. Res. 1985, 25, 21–31. [Google Scholar] [CrossRef]
- Schmucker, C.; Schaeffel, F. A paraxial schematic eye model for the growing c57bl/6 mouse. Vis. Res. 2004, 44, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.R.; de Sevilla Muller, L.P.; Brecha, N.C. The rna binding protein rbpms is a selective marker of ganglion cells in the mammalian retina. J. Comp. Neurol. 2014, 522, 1411–1443. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, T.-H.; Musada, G.R.; Romano, G.L.; Bolton, E.; Porciatti, V. Anesthetic Preconditioning as Endogenous Neuroprotection in Glaucoma. Int. J. Mol. Sci. 2018, 19, 237. https://doi.org/10.3390/ijms19010237
Chou T-H, Musada GR, Romano GL, Bolton E, Porciatti V. Anesthetic Preconditioning as Endogenous Neuroprotection in Glaucoma. International Journal of Molecular Sciences. 2018; 19(1):237. https://doi.org/10.3390/ijms19010237
Chicago/Turabian StyleChou, Tsung-Han, Ganeswara Rao Musada, Giovanni Luca Romano, Elizabeth Bolton, and Vittorio Porciatti. 2018. "Anesthetic Preconditioning as Endogenous Neuroprotection in Glaucoma" International Journal of Molecular Sciences 19, no. 1: 237. https://doi.org/10.3390/ijms19010237
APA StyleChou, T.-H., Musada, G. R., Romano, G. L., Bolton, E., & Porciatti, V. (2018). Anesthetic Preconditioning as Endogenous Neuroprotection in Glaucoma. International Journal of Molecular Sciences, 19(1), 237. https://doi.org/10.3390/ijms19010237