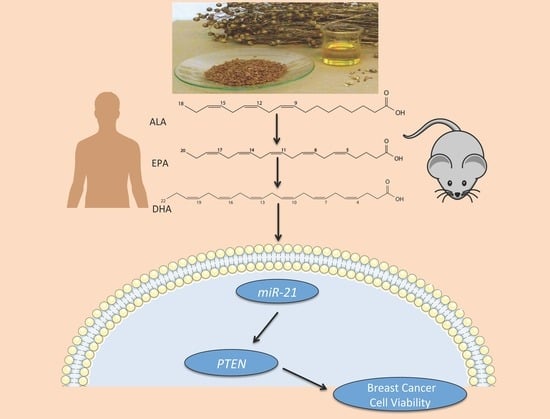
Omega-3 Polyunsaturated Fatty Acids Time-Dependently Reduce Cell Viability and Oncogenic MicroRNA-21 Expression in Estrogen Receptor-Positive Breast Cancer Cells (MCF-7)
Abstract
:1. Introduction
2. Results
2.1. Effect of ALA Alone or Combined with EPA and DHA on Cell Viability after 1, 3, 24, 48 and 96 h Treatment
2.2. Effect of ALA Alone or Combined with EPA and DHA on miR-21 Expression at Different Time Points
2.3. Effect of ALA Alone or Combined with EPA and DHA on PTEN Gene and Protein Expression after 12 and 24 h Treatment
3. Discussion
4. Materials and Methods
4.1. Cell Line, Cell Culture and Treatment Medium
4.2. Trypan Blue Exclusion Assay for Cell Viability
4.3. RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
4.4. Protein Biomarker Expression
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ALA | Alpha-linolenic acid |
| AnR | Animal fatty acid ratio treatment (ALA:EPA:DHA molar ratios in the blood) |
| BC | Breast cancer |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| FO | Fish oil |
| FSO | Flaxseed oil |
| HuR | Human fatty acid ratio treatment (ALA:EPA:DHA molar ratios in the blood) |
| miRNA | MicroRNA |
| n-3 PUFA | Omega-3 polyunsaturated fatty acid |
Appendix A

References
- Global Burden of Disease Cancer Collaboration. The global burden of cancer 2013. JAMA Oncol. 2015, 1, 505–527. [Google Scholar] [CrossRef]
- World Health Organization. Breast Cancer: Prevention and Control. Available online: http://www.who.int/cancer/detection/breastcancer/en/ (accessed on 21 May 2017).
- Witt, C.M.; Cardoso, M.J. Complementary and integrative medicine for breast cancer patients—Evidence based practical recommendations. Breast 2016, 28, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.G.; Taylor, A.G. Use of complementary therapies for cancer symptom management: Results of the 2007 national health interview survey. J. Altern. Complement. Med. 2012, 18, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Truan, J.S.; Chen, J.M.; Thompson, L.U. Flaxseed oil reduces the growth of human breast tumors (MCF-7) at high levels of circulating estrogen. Mol. Nutr. Food Res. 2010, 54, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.M.; Ma, D.W. Are all polyunsaturated fatty acids created equal? Lipids Health Dis. 2009, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Kaul, N.; Kreml, R.; Austria, J.A.; Richard, M.N.; Edel, A.L.; Dibrov, E.; Hirono, S.; Zettler, M.E.; Pierce, G.N. A comparison of fish oil, flaxseed oil and hempseed oil supplementation on selected parameters of cardivascular health in healthy volunteers. J. Am. Coll. Nutr. 2013, 27, 51–58. [Google Scholar] [CrossRef]
- Mason, J.K.; Klaire, S.; Kharotia, S.; Wiggins, A.K.; Thompson, L.U. α-linolenic acid and docosahexaenoic acid, alone and combined with trastuzumab, reduce HER2-overexpressing breast cancer cell growth but differentially regulate HER2 signaling pathways. Lipids Health Dis. 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, A.K.; Kharotia, S.; Mason, J.K.; Thompson, L.U. Alpha-linolenic acid reduces growth of both triple-negative and luminal breast cancer cells in high and low estrogen environments. Nutr. Cancer 2015, 278, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Chamras, H.; Ardashian, A.; Heber, D.; Glaspy, J.A. Fatty acid modulation of MCF-7 human breast cancer cell proliferation, apoptosis and differentiation. J. Nutr. Biochem. 2002, 13, 711–716. [Google Scholar] [CrossRef]
- Motawi, T.M.K.; Sadik, N.A.H.; Shaker, O.G.; El Masry, M.R.; Mohareb, F. Study of microRNAs-21-221 as potential breast cancer biomarkers in Egyptian women. Gene 2016, 590, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Jin, X.; Zhen, Y.; Hu, P. MicroRNA regulates estrogen receptor alpha in breast cancer metastasis. J. Cancer Res. Ther. Oncol. 2014, 2, 1–6. [Google Scholar]
- Gao, J.; Zhang, Q.; Xu, J.; Guo, L.; Li, X. Clinical significance of serum miR-21 in breast cancer compared with CA153 and CEA. Chin. J. Cancer Res. 2013, 25, 743–748. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, T.A.; Schwartz, G.N.; Calderone, H.M.; Graveel, C.R.; Winn, M.E.; Hostetter, G.; Wells, W.A.; Sempere, L.F. Stromal expression of miR-21 identifies high-risk group in triple-negative breast cancer. Am. J. Pathol. 2014, 184, 3217–3225. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.X.; Huang, X.F.; Shao, Q.; Huang, M.Y.; Deng, L.; Wu, Q.L.; Zeng, Y.X.; Shao, J.Y. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA 2008, 14, 2348–2360. [Google Scholar] [CrossRef] [PubMed]
- Mandal, C.C.; Ghosh-Choudhury, T.; Dey, N.; Choudhury, G.G.; Ghosh-Choudhury, N. miR-21 is targeted by omega-3 polyunsaturated fatty acid to regulate breast tumor CSF-1 expression. Carcinogenesis 2012, 33, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.A.; Wang, N.; Shah, M.S.; Lupton, J.R.; Ivanov, I.; Chapkin, R.S. n-3 Polyunsaturated fatty acids modulate carcinogen-directed non-coding microRNA signatures in rat colon. Carcinogenesis 2009, 30, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Bar, N.; Jayavelu, N.D. Reconstruction of temporal activity of microRNAs form gene expression data in breast cancer cell line. BMC Genom. 2015, 16, 1077. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.S.; Wang, P.; Yamabe, N.; Fukui, M.; Jay, T.; Zhu, B.T. Docosahexaenoic acid induces apoptosis in MCF-7 cells in vitro and in vivo via reactive oxygen species formation and caspase 8 activation. PLoS ONE 2010, 5, e10296. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Rawat, A.K.; Sammi, S.R.; Devi, U.; Singh, M.; Gautam, S.; Yadav, R.K.; Rawat, J.K.; Singh, L.; Ansari, M.N.; et al. Alpha-linolenic acid stabilizes HIF-1 α and downregulates FASN to promote mitochondrial apoptosis for mammary gland chemoprevention. Oncotarget 2017, 8, 70049–70071. [Google Scholar] [CrossRef] [PubMed]
- Mason-Ennis, J.K.; LeMay-Nedjelski, L.P.; Wiggins, A.K.A.; Thompson, L.U. Exploration of mechanisms of alpha-linolenic acid in reducing the growth of oestrogen receptor positive breast cancer cells (MCF-7). J. Funct. Foods 2016, 24, 513–519. [Google Scholar] [CrossRef]
- Grammatikos, S.I.; Subbaiah, P.V.; Victor, T.A.; Miller, W.M. n-3 and n-6 fatty acid processing and growth effects in neoplastic and non-cancerous human mammary epithelial cell lines. Br. J. Cancer 1994, 70, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Corsetto, P.A.; Montorfano, G.; Zava, S.; Jovenitti, I.E.; Cremona, A.; Berra, B.; Rizzo, A.M. Effects of n-3 PUFAs on breast cancer cells through their incorporation in plasma membrane. Lipids Health Dis. 2010, 10, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Blanckaert, V.; Ulmann, L.; Mimouni, V.; Antol, J.; Brancquart, L.; Chénais, B. Docosahexaenoic acid intake decreases proliferation, increases apoptosis and decreases the invasive potential of the human breast carcinoma cell line MDA-MB-231. Int. J. Oncol. 2010, 36, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Suburu, J.; Chen, H.; Chen, Y.Q. Mechanisms of omega-3 polyunsaturated fatty acids in prostate cancer prevention. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Chenais, B.; Blanckaert, V. The Janus face of lipids in human breast cancer: How polyunsaturated Fatty acids affect tumor cell hallmarks. Int. J. Breast Cancer 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Chini, B.; Parenti, M. G-protein coupled receptors in lipid rafts and caveolae: How, when and why do they go there? J. Mol. Endocrinol. 2004, 32, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Turk, H.F.; Chapkin, R.S. Membrane lipid raft organization is uniquely modified by n-3 polyunsaturated acids. Prostagland. Leuk. Essent. Fat. Acids 2013, 88, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Babina, I.S.; Donatello, S.; Nabi, I.R.; Hopkins, A.M. Lipid rafts as Master Regulators of Breast Cancer Function. In Breast Cancer-Carcinogenesis, Cell Growth and Signaling Pathways; Gunduz, M., Ed.; Intech Press: Rijeka, Croatia, 2011; pp. 401–428. [Google Scholar] [CrossRef]
- Martini, M.; De Santis, M.C.; Braccini, L.; Gulluni, F.; Hirsch, E. PI3K/Akt signaling pathway and cancer: And updated review. Ann. Med. 2014, 46, 372–383. [Google Scholar] [CrossRef] [PubMed]
- D’Eliseo, D.; Velotti, F. Omega-3 fatty acids and cancer cell cytotoxicity: Implications for multi-targeted cancer therapy. J. Clin. Med. 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, H.D.; Park, E.; Chon, J.W.; Park, Y.K. Growth-inhibitory and proapoptotic effects of alpha-linolenic acid on estrogen-positive breast cancer cells. Ann. N. Y. Acad. Sci. 2009, 1171, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Hu, G.; Gong, A.Y.; Chen, X.M. Binding of NF-kappaB p65 subunit to the promoter elements is involved in LPS-induced transactivation of miRNA genes in human biliary epithelial cells. Nucleic Acids Res. 2010, 38, 3222–3232. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Chen, G.; Cui, Q. Towards the understanding of microRNA and environmental factor interactions and their relationships to human diseases. Sci. Rep. 2012, 2, srep00318. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xu, C.; Guan, J.; Ping, Y.; Fan, H.; Li, Y.; Zhao, H.; Li, X. Discovering dysfunction of multiple microRNAs cooperation in disease by a conserved microRNA co-expression network. PLoS ONE 2012, 7, e32201. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, U.; Lai, X.; Wolkenhauer, O.; Vera, J.; Gupta, S.K. Cooperative gene regulation by microRNA pairs and their identification using a computational workflow. Nucleic Acids Res. 2014, 42, 7539–7552. [Google Scholar] [CrossRef] [PubMed]
- Field, C.J.; Schley, P.D. Evidence for potential mechanisms for the effect of conjugated linoleic acid on tumour metabolism and immune function: Lessons from n-3 fatty acids. Am. J. Clin. Nutr. 2004, 79, 1190S–1198S. [Google Scholar] [PubMed]
- Tsoukas, M.A.; Ko, B.; Witte, T.R.; Dincer, F.; Hardman, W.E.; Mantzoros, C.S. Dietary walnut suppression of colorectal cancer in mice: Mediation by miRNA patterns and fatty acid incorporation. J. Nutr. Biochem. 2015, 26, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Piccinoni, E.; Merendino, N.; Calviello, G. Dietary polyunsaturated fatty acids as inducers of apoptosis: Implications for cancer. Apoptosis 2009, 14, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Wickramasinghe, N.S.; Manavalan, T.T.; Dougherty, S.M.; Riggs, K.A.; Li, Y.; Klinge, C.M. Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells. Nucleic Acids Res. 2009, 37, 2584–2595. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhu, Z.; McGinley, J.N.; El Bayoumy, K.; Manni, A.; Thompson, H.J. Identification of a molecular signature underlying inhibition of mammary carcinoma growth by dietary n-3 fatty acids. Cancer Res. 2012, 72, 3795–3806. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Gallon, D.; Vissac-Sabatier, C.; Antoine-Vincent, D.; Rio, P.G.; Maurizis, J.C.; Fustier, P.; Bignon, Y.J. Differential effects of n-3 and n-6 polyunsaturated fatty acids on BRCA1 and BRCA2 gene expression in breast cell lines. Br. J. Nutr. 2002, 87, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, M.-L.; Mahéo, K.; Barascu, A.; Goupille, C.; de Latour, M.P.; Bougnoux, P.; Rio, P.G. Increased BRCA1 protein in mammary tumours of rats fed marine ω-3 fatty acids. Oncol. Rep. 2007, 17, 713–719. [Google Scholar] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
LeMay-Nedjelski, L.; Mason-Ennis, J.K.; Taibi, A.; Comelli, E.M.; Thompson, L.U. Omega-3 Polyunsaturated Fatty Acids Time-Dependently Reduce Cell Viability and Oncogenic MicroRNA-21 Expression in Estrogen Receptor-Positive Breast Cancer Cells (MCF-7). Int. J. Mol. Sci. 2018, 19, 244. https://doi.org/10.3390/ijms19010244
LeMay-Nedjelski L, Mason-Ennis JK, Taibi A, Comelli EM, Thompson LU. Omega-3 Polyunsaturated Fatty Acids Time-Dependently Reduce Cell Viability and Oncogenic MicroRNA-21 Expression in Estrogen Receptor-Positive Breast Cancer Cells (MCF-7). International Journal of Molecular Sciences. 2018; 19(1):244. https://doi.org/10.3390/ijms19010244
Chicago/Turabian StyleLeMay-Nedjelski, Lauren, Julie K. Mason-Ennis, Amel Taibi, Elena M. Comelli, and Lilian U. Thompson. 2018. "Omega-3 Polyunsaturated Fatty Acids Time-Dependently Reduce Cell Viability and Oncogenic MicroRNA-21 Expression in Estrogen Receptor-Positive Breast Cancer Cells (MCF-7)" International Journal of Molecular Sciences 19, no. 1: 244. https://doi.org/10.3390/ijms19010244
APA StyleLeMay-Nedjelski, L., Mason-Ennis, J. K., Taibi, A., Comelli, E. M., & Thompson, L. U. (2018). Omega-3 Polyunsaturated Fatty Acids Time-Dependently Reduce Cell Viability and Oncogenic MicroRNA-21 Expression in Estrogen Receptor-Positive Breast Cancer Cells (MCF-7). International Journal of Molecular Sciences, 19(1), 244. https://doi.org/10.3390/ijms19010244