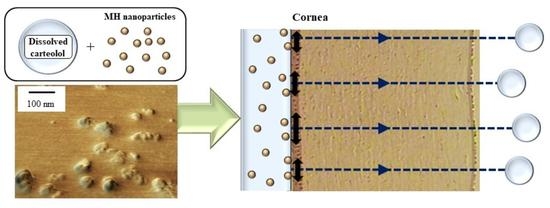
Enhancement in Corneal Permeability of Dissolved Carteolol by Its Combination with Magnesium Hydroxide Nanoparticles
Abstract
:1. Introduction
2. Results
2.1. Changes in the Ocular Surface by the Instillation of Carteolol/MH Microparticle and Carteolol/MH Nanoparticle Fixed Combinations
2.2. Enhancement of Transcorneal Penetration and IOP-Reducing Effect of Carteolol in Combination with MH Nanoparticles
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Preparation of CMFC Ophthalmic Formulations
4.4. Solubility of MH in Lacrimal Fluid
4.5. Measurement of Lacrimal Fluid and Corneal Toxicity by Instillation of nCMFC
4.6. In Vitro Transcorneal Penetration of CMFC
4.7. In Vivo Transcorneal Penetration of CMFC
4.8. Measurement of Intraocular Pressure in Rabbits
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Abs | absorbance |
| AUC | area under the carteolol concentration-time curve |
| BAC | benzalkonium chloride |
| carteolol | carteolol hydrochloride |
| CMFC | carteolol/MH nanoparticle fixed combinations |
| DDS | drug delivery systems |
| IOP | intraocular pressure |
| mannitol | d-mannitol |
| MC | methylcellulose |
| MH | magnesium hydroxide |
| MRT | the mean residence time |
| S.E.M. | standard error of the mean |
References
- American Academy of Ophthalmology. Primary Open-Angle Glaucoma Preferred Practice Patterns; American Academy of Ophthalmology: San Francisco, CA, USA, 2010. [Google Scholar]
- National Institute for Health and Care Excellence. Glaucoma: Dianosis and Management of Chronic Open Angle Glaucoma and Ocular Hypertension; National Collaborating Centre for Acute Care: London, UK, 2009.
- Boland, M.V.; Ervin, A.M.; Friedman, D.; Jampel, H.; Hawkins, B.; Volenweider, D.; Chelladurai, Y.; Ward, D.; Suarez-Cuervo, C.; Robinson, K.A. Treatment for Glaucoma: Comparative Effectiveness; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2012.
- Boland, M.V.; Ervin, A.M.; Friedman, D.S.; Jampel, H.D.; Hawkins, B.S.; Vollenweider, D.; Chelladurai, Y.; Ward, D.; Suarez-Cuervo, C.; Robinson, K.A. Comparative effectiveness of treatments for open-angle glaucoma: A systematic review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 15, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.M.; Wollstein, G.; Schuman, J.S. Clinical Utility of Optical Coherence Tomography in Glaucoma. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT556–OCT567. [Google Scholar] [CrossRef] [PubMed]
- Scripsema, N.K.; Garcia, P.M.; Bavier, R.D.; Chui, T.Y.; Krawitz, B.D.; Mo, S.; Agemy, S.A.; Xu, L.; Lin, Y.B.; Panarelli, J.F.; et al. Optical Coherence Tomography Angiography Analysis of Perfused Peripapillary Capillaries in Primary Open-Angle Glaucoma and Normal-Tension Glaucoma. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT611–OCT620. [Google Scholar] [CrossRef] [PubMed]
- Janczewski, P.; Boulanger, C.; Iqbal, A.; Vanhoutte, P.M. Endothelium-dependent effects of carteolol. J. Pharmacol. Exp. Ther. 1988, 247, 590–595. [Google Scholar] [PubMed]
- Man, I.A.V.; Schalekamp, M.A. How intrinsic sympathomimetic activity modulates the haemodynamic responses to beta-adrenoreceptor antagonists: A clue to the nature of their antihypertensive mechanism. Br. J. Clin. Pharmacol. 1982, 13, 245S–257S. [Google Scholar]
- Stewart, W.C. Carteolol, an ophthalmic beta-adrenergic blocker with intrinsic sympathomimetic activity. J. Glaucoma 1994, 3, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Lindsley, K.; Rouse, B.; Hong, H.; Shi, Q.; Friedman, D.S.; Wormald, R.; Dickersin, K. Comparative Effectiveness of First-Line Medications for Primary Open-Angle Glaucoma: A Systematic Review and Network Meta-analysis. Ophthalmology 2016, 123, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Montanari, P.; Marangoni, P.; Oldani, A.; Ratiglia, R.; Raiteri, M.; Berardinelli, L. Color Doppler imaging study in patients with primary open-angle glaucoma treated with timolol 0.5% and carteolol 2%. Eur. J. Ophthalmol. 2001, 11, 240–244. [Google Scholar] [PubMed]
- Altan-Yaycioglu, R.; Turker, G.; Akdol, S.; Acunas, G.; Izgi, B. The effects of beta-blockers on ocular blood flow in patients with primary open angle glaucoma: A color Doppler imaging study. Eur. J. Ophthalmol. 2001, 11, 37–46. [Google Scholar] [PubMed]
- Tamaki, Y.; Araie, M.; Tomita, K.; Tomidokoro, A.; Nagahara, M. Effects of topical adrenergic agents on tissue circulation in rabbit and human optic nerve head evaluated with laser speckle tissue circulation analyzer. Surv. Ophthalmol. 1997, 42, 52–62. [Google Scholar] [CrossRef]
- Tamaki, Y.; Araie, M.; Tomita, K.; Nagahara, M.; Tomidokoro, A. Effect of topical beta-blockers on tissue blood flow in the human optic nerve head. Curr. Eye Res. 1997, 16, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Deguchi, S.; Otake, H.; Hiramatsu, N.; Yamamoto, N. Therapeutic Effect of Cilostazol Ophthalmic Nanodispersions on Retinal Dysfunction in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2017, 18, 1971. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yoshioka, C.; Tanabe, W.; Tanino, T.; Ito, Y.; Okamoto, N.; Shimomura, Y. Effects of Ophthalmic Formulations containing Cilostazol Nanoparticles on Retinal Vasoconstriction in Rats Injected with Endothelin-1. Pharm. Anal. Acta 2015, 6, 4. [Google Scholar]
- Okamoto, N.; Ito, Y.; Nagai, N.; Murao, T.; Takiguchi, Y.; Kurimoto, T.; Mimura, O. Preparation of Ophthalmic Formulations Containing Cilostazol as an Anti-glaucoma Agent and Improvement in Its Permeability through the Rabbit Cornea. J. Oleo Sci. 2010, 59, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Mano, Y.; Ito, Y. An Ophthalmic Formulation of Disulfiram Nanoparticles Prolongs Drug Residence Time in Lens. Biol. Pharm. Bull. 2016, 39, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yoshioka, C.; Mano, Y.; Tanabe, W.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation of disulfiram prolongs corneal residence time of the drug and reduces intraocular pressure. Exp. Eye Res. 2015, 132, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation reduces the corneal toxicity of indomethacin eye drops and enhances its corneal permeability. Toxicology 2014, 319, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Nakazawa, Y.; Ito, Y.; Kanai, K.; Okamoto, N.; Shimomura, Y. A Nanoparticle-Based Ophthalmic Formulation of Dexamethasone Enhances Corneal Permeability of the Drug and Prolongs Its Corneal Residence Time. Biol. Pharm. Bull. 2017, 40, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ono, H.; Hashino, M.; Ito, Y.; Okamoto, N.; Shimomura, Y. Improved corneal toxicity and permeability of tranilast by the preparation of ophthalmic formulations containing its nanoparticles. J. Oleo Sci. 2014, 63, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ogata, F.; Otake, H.; Kawasaki, N.; Nakazawa, Y.; Kanai, K.; Okamoto, N.; Shimomura, Y. Co-instillation of nano-solid magnesium hydroxide enhances corneal permeability of dissolved timolol. Exp. Eye Res. 2017, 165, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Nagaoka, T.; Takahashi, A.; Sato, E.; Yoshida, A. Effects of topical carteolol on retinal arterial blood flow in primary open-angle glaucoma patients. Jpn. J. Ophthalmol. 2012, 56, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Henness, S.; Swainston, H.T.; Keating, G.M. Ocular carteolol: A review of its use in the management of glaucoma and ocular hypertension. Drugs Aging 2007, 24, 509–528. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yoshioka, C.; Tanino, T.; Ito, Y.; Okamoto, N.; Shimomura, Y. Decrease in Corneal Damage due to Benzalkonium Chloride by the Addition of Mannitol into Timolol Maleate Eye Drops. J. Oleo Sci. 2015, 64, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Dacus, A.C.; Bartels, S.P. Adrenergic mechanism in circadian elevation of intraocular pressure in rabbits. Investig. Ophthalmol. Vis. Sci. 1991, 32, 2178–2183. [Google Scholar]
- Kiuchi, Y.; Yoshitomi, T.; Gregory, D.S. Do alpha-adrenergic receptors participate in control of the circadian rhythm of IOP? Investig. Ophthalmol. Vis. Sci. 1992, 33, 3186–3194. [Google Scholar]
- Mirza, G.E.; Karakucuk, S.; Temel, E. Comparison of the effects of 0.5% timolol maleate, 2% carteolol hydrochloride, and 0.3% metipranolol on intraocular pressure and perimetry findings and evaluation of their ocular and systemic effects. J. Glaucoma 2000, 9, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.C.; Shields, M.B.; Allen, R.C.; Lewis, R.A.; Cohen, J.S.; Hoskins, H.D.; Hetherington, J.N.; Bahr, R.L.; Noblin, J.E.; Delehanty, J.T. A 3-month comparison of 1% and 2% carteolol and 0.5% timolol in open-angle glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 1991, 229, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.C.; Cohen, J.S.; Netland, P.A.; Weiss, H.; Nussbaum, L.L. Efficacy of carteolol hydrochloride 1% vs. timolol maleate 0.5% in patients with increased intraocular pressure. Nocturnal Investigation of Glaucoma Hemodynamics Trial Study Group. Am. J. Ophthalmol. 1997, 124, 498–505. [Google Scholar] [CrossRef]
- Diebold, Y.; Calonge, M. Applications of nanoparticles in ophthalmology. Prog. Retin. Eye Res. 2010, 29, 596–609. [Google Scholar] [CrossRef] [PubMed]






| Formulation | Jc (nmol·cm−2·min−1) | kp (×10−4·min−1) | km (×10−3) | τ (min) | D (×10−5·cm2·min−1) |
|---|---|---|---|---|---|
| CRT-solution | 9.7 ± 1.2 | 6.4 ± 0.8 | 1.0 ± 0.2 | 17.1 ± 2.1 | 3.8 ± 0.2 |
| mCMFC | 9.9 ± 1.3 | 6.0 ± 1.0 | 1.1 ± 0.2 | 16.7 ± 1.8 | 3.9 ± 0.6 |
| nCMFC | 17.6 ± 1.7 *1,2 | 11.6 ± 1.1 *1,2 | 0.2 ± 0.1 *1,2 | 1.9 ± 0.4 *1,2 | 33.1 ± 4.7 *1,2 |
| Formulation | AUC (mM·min) | MRT (min) |
|---|---|---|
| CRT-solution | 0.98 ± 0.14 | 65.6 ± 0.5 |
| mCMFC | 1.65 ± 0.23 | 62.7 ± 3.1 |
| nCMFC | 9.45 ± 1.71 *1,2 | 60.6 ± 2.3 |
| Formulation | MH Particles | Carteolol | MC | BAC | Mannitol | Treatment |
|---|---|---|---|---|---|---|
| CRT-solution | - | 1% | 0.5% | 0.001% | 0.5% | - |
| mCMFC | 0.01% | 1% | 0.5% | 0.001% | 0.5% | - |
| nCMFC | 0.01% | 1% | 0.5% | 0.001% | 0.5% | Bead mill |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagai, N.; Yamaoka, S.; Fukuoka, Y.; Ishii, M.; Otake, H.; Kanai, K.; Okamoto, N.; Shimomura, Y. Enhancement in Corneal Permeability of Dissolved Carteolol by Its Combination with Magnesium Hydroxide Nanoparticles. Int. J. Mol. Sci. 2018, 19, 282. https://doi.org/10.3390/ijms19010282
Nagai N, Yamaoka S, Fukuoka Y, Ishii M, Otake H, Kanai K, Okamoto N, Shimomura Y. Enhancement in Corneal Permeability of Dissolved Carteolol by Its Combination with Magnesium Hydroxide Nanoparticles. International Journal of Molecular Sciences. 2018; 19(1):282. https://doi.org/10.3390/ijms19010282
Chicago/Turabian StyleNagai, Noriaki, Sakie Yamaoka, Yuya Fukuoka, Miyu Ishii, Hiroko Otake, Kazutaka Kanai, Norio Okamoto, and Yoshikazu Shimomura. 2018. "Enhancement in Corneal Permeability of Dissolved Carteolol by Its Combination with Magnesium Hydroxide Nanoparticles" International Journal of Molecular Sciences 19, no. 1: 282. https://doi.org/10.3390/ijms19010282
APA StyleNagai, N., Yamaoka, S., Fukuoka, Y., Ishii, M., Otake, H., Kanai, K., Okamoto, N., & Shimomura, Y. (2018). Enhancement in Corneal Permeability of Dissolved Carteolol by Its Combination with Magnesium Hydroxide Nanoparticles. International Journal of Molecular Sciences, 19(1), 282. https://doi.org/10.3390/ijms19010282