Affinity Purification and Comparative Biosensor Analysis of Citrulline-Peptide-Specific Antibodies in Rheumatoid Arthritis
Abstract
:1. Introduction
2. Results
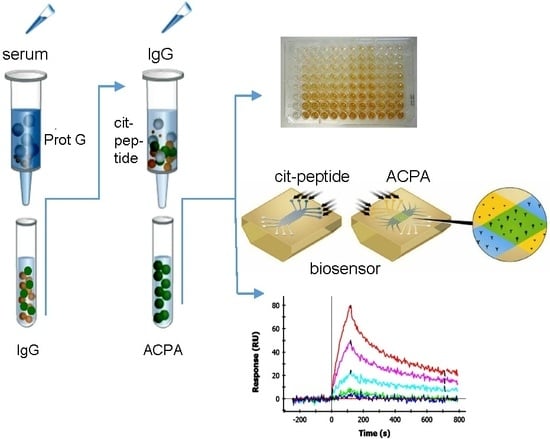
2.1. Affinity Purification of ACPA
2.2. Cross-Reaction of Affinity-Purified ACPA IgG Fractions
2.3. Analysis of the Multi-Epitope Peptide by ELISA Screening of RA Sera
2.4. SPR Analysis of Affinity-Purified Antibodies and Serum Antibodies of RA Patients
2.4.1. KD Determination of Serum Antibodies from RA Patients Using Immobilized Cit-Peptide on GLH Chip
2.4.2. Binding of Serum IgG to the Biotinylated Peptides Immobilized on Neutravidin-Coated Chip (NLC)
2.5. Selective Depletion of Autoantibody Producing B Cells Ex Vivo by the Multi-Epitope Peptide Covalently Coupled to Poly (Lactic-Co-Glycolic Acid) (PLGA) Nanoparticles (NP)
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. ACPA Affinity Purification
4.3. ELISA
4.4. SPR Analysis
4.5. Peptide Synthesis
4.6. Preparation and Characterization of Bifunctional PLGA NPs
4.7. Detection of Cit-Peptide-Specific Antibody Producing Cells
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACPA | Anti-Citrullinated Protein/peptide Antibodies |
| Cit | Citrulline |
| DAS | Disease Activity Score |
| EBNA | Epstein-Barr virus Nuclear Antigen |
| EBV | Epstein-Barr virus |
| IVIG | Intravenous Immunoglobulin |
| NET | Neutrophil Extracellular Trap |
| NHS | Normal Human Sera |
| NP | Nanoparticles |
| PBMC | Peripheral Blood Mononuclear Cells |
| PLGA | Poly(lactic-co-glycolic acid) |
| RA | Rheumatoid arthritis |
| SPR | Surface Plasmon Resonance |
References
- Gabriel, S.E. The Epidemiology of Rheumatoid Arthritis. Rheum. Dis. Clin. N. Am. 2001, 27, 269–281. [Google Scholar] [CrossRef]
- Yamada, R.; Suzuki, A.; Chang, X.; Yamamoto, K. Citrullinated Proteins in Rheumatoid Arthritis. Front. Biosci. 2005, 10, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Vossenaar, E.R.; Zendman, A.J.; van Venrooij, W.J.; Pruijn, G.J. Pad, a Growing Family of Citrullinating Enzymes: Genes, Features and Involvement in Disease. Bioessays 2003, 25, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Yamada, R.; Suzuki, A.; Chang, X.; Yamamoto, K. Peptidylarginine Deiminase Type 4: Identification of a Rheumatoid Arthritis-Susceptible Gene. Trends Mol. Med. 2003, 9, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Tarcsa, E.; Marekov, L.N.; Mei, G.; Melino, G.; Lee, S.C.; Steinert, P.M. Protein Unfolding by Peptidylarginine Deiminase. Substrate Specificity and Structural Relationships of the Natural Substrates Trichohyalin and Filaggrin. J. Biol. Chem. 1996, 271, 30709–30716. [Google Scholar] [CrossRef] [PubMed]
- Gyorgy, B.; Toth, E.; Tarcsa, E.; Falus, A.; Buzas, E.I. Citrullination: A Posttranslational Modification in Health and Disease. Int. J. Biochem. Cell Biol. 2006, 38, 1662–1677. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, H.; Sehnert, B.; Bockermann, R.; Engstrom, A.; Kalden, J.R.; Holmdahl, R. Humoral Immune Response to Citrullinated Collagen Type Ii Determinants in Early Rheumatoid Arthritis. Eur. J. Immunol. 2005, 35, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Sebbag, M.; Moinard, N.; Auger, I.; Clavel, C.; Arnaud, J.; Nogueira, L.; Roudier, J.; Serre, G. Epitopes of Human Fibrin Recognized by the Rheumatoid Arthritis-Specific Autoantibodies to Citrullinated Proteins. Eur. J. Immunol. 2006, 36, 2250–2263. [Google Scholar] [CrossRef] [PubMed]
- Masson-Bessiere, C.; Sebbag, M.; Girbal-Neuhauser, E.; Nogueira, L.; Vincent, C.; Senshu, T.; Serre, G. The Major Synovial Targets of the Rheumatoid Arthritis-Specific Antifilaggrin Autoantibodies Are Deiminated Forms of the Alpha- and Beta-Chains of Fibrin. J. Immunol. 2001, 166, 4177–4184. [Google Scholar] [CrossRef] [PubMed]
- Bang, H.; Egerer, K.; Gauliard, A.; Luthke, K.; Rudolph, P.E.; Fredenhagen, G.; Berg, W.; Feist, E.; Burmester, G.R. Mutation and Citrullination Modifies Vimentin to a Novel Autoantigen for Rheumatoid Arthritis. Arthritis Rheum. 2007, 56, 2503–2511. [Google Scholar] [CrossRef] [PubMed]
- Vossenaar, E.R.; Despres, N.; Lapointe, E.; van der Heijden, A.; Lora, M.; Senshu, T.; van Venrooij, W.J.; Menard, H.A. Rheumatoid Arthritis Specific Anti-Sa Antibodies Target Citrullinated Vimentin. Arthritis Res. Ther. 2004, 6, R142–R150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mor-Vaknin, N.; Punturieri, A.; Sitwala, K.; Markovitz, D.M. Vimentin Is Secreted by Activated Macrophages. Nat. Cell Biol. 2003, 5, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Snir, O.; Widhe, M.; Hermansson, M.; von Spee, C.; Lindberg, J.; Hensen, S.; Lundberg, K.; Engstrom, A.; Venables, P.J.; Toes, R.E.; et al. Antibodies to Several Citrullinated Antigens Are Enriched in the Joints of Rheumatoid Arthritis Patients. Arthritis Rheum. 2010, 62, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Clavel, C.; Nogueira, L.; Laurent, L.; Iobagiu, C.; Vincent, C.; Sebbag, M.; Serre, G. Induction of Macrophage Secretion of Tumor Necrosis Factor Alpha through Fcgamma Receptor Iia Engagement by Rheumatoid Arthritis-Specific Autoantibodies to Citrullinated Proteins Complexed with Fibrinogen. Arthritis Rheum. 2008, 58, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Sokolove, J.; Zhao, X.; Chandra, P.E.; Robinson, W.H. Immune Complexes Containing Citrullinated Fibrinogen Costimulate Macrophages Via Toll-Like Receptor 4 and Fcgamma Receptor. Arthritis Rheum. 2011, 63, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Moeez, S.; John, P.; Bhatti, A. Anti-Citrullinated Protein Antibodies: Role in Pathogenesis of Ra and Potential as a Diagnostic Tool. Rheumatol. Int. 2013, 33, 1669–1673. [Google Scholar] [CrossRef] [PubMed]
- Tilleman, K.; van Steendam, K.; Cantaert, T.; de Keyser, F.; Elewaut, D.; Deforce, D. Synovial Detection and Autoantibody Reactivity of Processed Citrullinated Isoforms of Vimentin in Inflammatory Arthritides. Rheumatology 2008, 47, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Van Steendam, K.; Tilleman, K.; Deforce, D. The Relevance of Citrullinated Vimentin in the Production of Antibodies against Citrullinated Proteins and the Pathogenesis of Rheumatoid Arthritis. Rheumatology 2011, 50, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Kinloch, A.; Tatzer, V.; Wait, R.; Peston, D.; Lundberg, K.; Donatien, P.; Moyes, D.; Taylor, P.C.; Venables, P.J. Identification of Citrullinated Alpha-Enolase as a Candidate Autoantigen in Rheumatoid Arthritis. Arthritis Res. Ther. 2005, 7, R1421–R1429. [Google Scholar] [CrossRef] [PubMed]
- Pratesi, F.; Tommasi, C.; Anzilotti, C.; Chimenti, D.; Migliorini, P. Deiminated Epstein-Barr Virus Nuclear Antigen 1 Is a Target of Anti-Citrullinated Protein Antibodies in Rheumatoid Arthritis. Arthritis Rheum. 2006, 54, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Sanmarti, R.; Graell, E.; Perez, M.L.; Ercilla, G.; Vinas, O.; Gomez-Puerta, J.A.; Gratacos, J.; Balsa, A.; Gomara, M.J.; Larrosa, M.; et al. Diagnostic and Prognostic Value of Antibodies against Chimeric Fibrin/Filaggrin Citrullinated Synthetic Peptides in Rheumatoid Arthritis. Arthritis Res. Ther. 2009, 11, R135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Wang, Y. Peptidylarginine Deiminases in Citrullination, Gene Regulation, Health and Pathogenesis. Biochim. Biophys. Acta 2013, 1829, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.Y.; Liao, Y.F.; Chang, W.H.; Liu, C.C.; Hsieh, M.C.; Hsu, P.C.; Tsay, G.J.; Hung, H.C. Overexpression of Peptidylarginine Deiminase Iv Features in Apoptosis of Haematopoietic Cells. Apoptosis 2006, 11, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Bawadekar, M.; Gendron-Fitzpatrick, A.; Rebernick, R.; Shim, D.; Warner, T.F.; Nicholas, A.P.; Lundblad, L.K.; Thompson, P.R.; Shelef, M.A. Tumor Necrosis Factor Alpha, Citrullination, and Peptidylarginine Deiminase 4 in Lung and Joint Inflammation. Arthritis Res. Ther. 2016, 18, 173. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, A.S.; Hemmers, S.; Arandjelovic, S.; Corr, M.; Mowen, K.A. Pad4 Is Not Essential for Disease in the K/Bxn Murine Autoantibody-Mediated Model of Arthritis. Arthritis Res. Ther. 2012, 14, R104. [Google Scholar] [CrossRef] [PubMed]
- Ioan-Facsinay, A.; el-Bannoudi, H.; Scherer, H.U.; van der Woude, D.; Menard, H.A.; Lora, M.; Trouw, L.A.; Huizinga, T.W.; Toes, R.E. Anti-Cyclic Citrullinated Peptide Antibodies Are a Collection of Anti-Citrullinated Protein Antibodies and Contain Overlapping and Non-Overlapping Reactivities. Ann. Rheum. Dis. 2011, 70, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Van Venrooij, W.J.; van Beers, J.J.; Pruijn, G.J. Anti-Ccp Antibodies: The Past, the Present and the Future. Nat. Rev. Rheumatol. 2011, 7, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Van de Stadt, L.A.; van Schouwenburg, P.A.; Bryde, S.; Kruithof, S.; van Schaardenburg, D.; Hamann, D.; Wolbink, G.; Rispens, T. Monoclonal Anti-Citrullinated Protein Antibodies Selected on Citrullinated Fibrinogen Have Distinct Targets with Different Cross-Reactivity Patterns. Rheumatology 2013, 52, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Goules, J.D.; Goules, A.V.; Tzioufas, A.G. Fine Specificity of Anti-Citrullinated Peptide Antibodies Discloses a Heterogeneous Antibody Population in Rheumatoid Arthritis. Clin. Exp. Immunol. 2013, 174, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Trier, N.H.; Leth, M.L.; Hansen, P.R.; Houen, G. Cross-Reactivity of a Human Igg(1) Anticitrullinated Fibrinogen Monoclonal Antibody to a Citrullinated Profilaggrin Peptide. Protein Sci. 2012, 21, 1929–1941. [Google Scholar] [CrossRef] [PubMed]
- Szarka, E.; Babos, F.; Magyar, A.; Huber, K.; Szittner, Z.; Papp, K.; Prechl, J.; Pozsgay, J.; Neer, Z.; Adori, M.; et al. Recognition of New Citrulline-Containing Peptide Epitopes by Autoantibodies Produced In Vivo and In Vitro by B Cells of Rheumatoid Arthritis Patients. Immunology 2014, 141, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Pozsgay, J.; Babos, F.; Uray, K.; Magyar, A.; Gyulai, G.; Kiss, E.; Nagy, G.; Rojkovich, B.; Hudecz, F.; Sarmay, G. In Vitro Eradication of Citrullinated Protein Specific B-Lymphocytes of Rheumatoid Arthritis Patients by Targeted Bifunctional Nanoparticles. Arthritis Res. Ther. 2016, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Schellekens, G.A.; de Jong, B.A.; van den Hoogen, F.H.; van de Putte, L.B.; van Venrooij, W.J. Citrulline Is an Essential Constituent of Antigenic Determinants Recognized by Rheumatoid Arthritis-Specific Autoantibodies. J. Clin. Investig. 1998, 101, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Iobagiu, C.; Magyar, A.; Nogueira, L.; Cornillet, M.; Sebbag, M.; Arnaud, J.; Hudecz, F.; Serre, G. The Antigen Specificity of the Rheumatoid Arthritis-Associated Acpa Directed to Citrullinated Fibrin Is Very Closely Restricted. J. Autoimmun. 2011, 37, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, K.; Kinloch, A.; Fisher, B.A.; Wegner, N.; Wait, R.; Charles, P.; Mikuls, T.R.; Venables, P.J. Antibodies to Citrullinated Alpha-Enolase Peptide 1 Are Specific for Rheumatoid Arthritis and Cross-React with Bacterial Enolase. Arthritis Rheum. 2008, 58, 3009–3019. [Google Scholar] [CrossRef] [PubMed]
- Pratesi, F.; Tommasi, C.; Anzilotti, C.; Puxeddu, I.; Sardano, E.; di Colo, G.; Migliorini, P. Antibodies to a New Viral Citrullinated Peptide, VCP2: Fine Specificity and Correlation with Anti-Cyclic Citrullinated Peptide (CCP) and Anti-VCP1 Antibodies. Clin. Exp. Immunol. 2011, 164, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Babos, F.; Szarka, E.; Nagy, G.; Majer, Z.; Sarmay, G.; Magyar, A.; Hudecz, F. Role of N- or C-Terminal Biotinylation in Autoantibody Recognition of Citrullin Containing Filaggrin Epitope Peptides in Rheumatoid Arthritis. Bioconjug. Chem. 2013, 24, 817–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ossipova, E.; Cerqueira, C.F.; Reed, E.; Kharlamova, N.; Israelsson, L.; Holmdahl, R.; Nandakumar, K.S.; Engstrom, M.; Harre, U.; Schett, G.; et al. Affinity Purified Anti-Citrullinated Protein/Peptide Antibodies Target Antigens Expressed in the Rheumatoid Joint. Arthritis Res. Ther. 2014, 16, R167. [Google Scholar] [CrossRef] [PubMed]
- Suwannalai, P.; Britsemmer, K.; Knevel, R.; Scherer, H.U.; Levarht, E.W.; Van der Helm-van Mil, A.H.; van Schaardenburg, D.; Huizinga, T.W.; Toes, R.E.; Trouw, L.A. Low-Avidity Anticitrullinated Protein Antibodies (ACPA) Are Associated with a Higher Rate of Joint Destruction in Rheumatoid Arthritis. Ann. Rheum. Dis. 2014, 73, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Susal, C.; Kirschfink, M.; Kropelin, M.; Daniel, V.; Opelz, G. Identification of Complement Activation Sites in Human Immunodeficiency Virus Type-1 Glycoprotein Gp120. Blood 1996, 87, 2329–2336. [Google Scholar] [PubMed]
- Rossi, G.; Real-Fernandez, F.; Panza, F.; Barbetti, F.; Pratesi, F.; Rovero, P.; Migliorini, P. Biosensor Analysis of Anti-Citrullinated Protein/Peptide Antibody Affinity. Anal. Biochem. 2014, 465, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Trier, N.H.; Dam, C.E.; Olsen, D.T.; Hansen, P.R.; Houen, G. Contribution of Peptide Backbone to Anti-Citrullinated Peptide Antibody Reactivity. PLoS ONE 2015, 10, e0144707. [Google Scholar] [CrossRef] [PubMed]
- Cornillet, M.; Sebbag, M.; Verrouil, E.; Magyar, A.; Babos, F.; Ruyssen-Witrand, A.; Hudecz, F.; Cantagrel, A.; Serre, G.; Nogueira, L. The Fibrin-Derived Citrullinated Peptide β60–74Cit60,72,74 Bears the Major ACPA Epitope Recognised by the Rheumatoid Arthritis-Specific Anticitrullinated Fibrinogen Autoantibodies and Anti-CCP2 Antibodies. Ann. Rheum. Dis. 2014, 73, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Pratesi, F.; Panza, F.; Paolini, I.; Petrelli, F.; Puxeddu, I.; Casigliani-Rabl, S.; Ancillotti, D.; Alcaro, C.; Rovero, P.; Migliorini, P. Fingerprinting of Anti-Citrullinated Protein Antibodies (ACPA): Specificity, Isotypes and Subclasses. Lupus 2015, 24, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Dam, C.E.; Houen, G.; Trier, N.H. The Dependency on Neighboring Amino Acids for Reactivity of Anti-Citrullinated Protein Antibodies to Citrullinated Proteins. Scand. J. Clin. Lab. Investig. 2016, 76, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Derksen, V.F.; Huizinga, T.W.J.; van der Woude, D. The Role of Autoantibodies in the Pathophysiology of Rheumatoid Arthritis. Semin. Immunopathol. 2017, 39, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Klareskog, L.; Catrina, A.I.; Paget, S. Rheumatoid Arthritis. Lancet 2009, 373, 659–672. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., III; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef] [PubMed]








| Peptides | Sequences | References |
|---|---|---|
| Filaggrin (306–326) | Ac-SHQESTXGXSXGRSGRSGSK-NH2 | [33] |
| Collagen II (359–369) | Ac-AXGLTGXPGDA-NH2 | [7] |
| Fibrin β (60–74) | H-XPAPPPISGGGYXAX-NH2 | [8,34] |
| Vimentin (65–77) | Ac-SAVRAXSSVPGVRK-NH2 | [10] |
| α-enolase (5–21) | Ac-KIHAXEIFDSXGNPTVE-NH2 | [35] |
| EBNA-2 (341–361) | Ac-GQSXGQSXGXGXGXGXGXGKG-NH2 | [36] |
| multi-epitope | H-Ttds-AXAXGSGSGXGXG-NH2 |
| Peptides | Filaggrin | Collagen II | Fibrin β | Vimentin | Multi-Epitope |
|---|---|---|---|---|---|
| filaggrin19 ACPA | 4.05 × 10−7 | 2.10 × 10−6 | 1.65 × 10−6 | nb * | 1.33 × 10−6 |
| collagen II ACPA | 5.32 × 10−8 | 6.10 × 10−8 | 8.44 × 10−8 | 1.31 × 10−7 | 7.17 × 10−8 |
| fibrin β ACPA | 3.56 × 10−7 | 1.21 × 10−6 | 8.34 × 10−7 | 3.86 × 10−7 | 2.75 × 10−7 |
| vimentin ACPA | 5.58 × 10−7 | nb | 1.65 × 10−6 | nb | nb |
| α-enolase ACPA | nb | 8.96 × 10−8 | 2.47 × 10−8 | nb | 2.94 × 10−8 |
| Peptide | Immobilization Buffer | Immobilized Peptide (RU) |
|---|---|---|
| Filaggrin (306–326) | sodium acetate pH 4 | 500 |
| Vimentin (65–77) | borate pH 9 | 5700 |
| Multi-epitope | borate pH 9.5 | 3100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szarka, E.; Aradi, P.; Huber, K.; Pozsgay, J.; Végh, L.; Magyar, A.; Gyulai, G.; Nagy, G.; Rojkovich, B.; Kiss, É.; et al. Affinity Purification and Comparative Biosensor Analysis of Citrulline-Peptide-Specific Antibodies in Rheumatoid Arthritis. Int. J. Mol. Sci. 2018, 19, 326. https://doi.org/10.3390/ijms19010326
Szarka E, Aradi P, Huber K, Pozsgay J, Végh L, Magyar A, Gyulai G, Nagy G, Rojkovich B, Kiss É, et al. Affinity Purification and Comparative Biosensor Analysis of Citrulline-Peptide-Specific Antibodies in Rheumatoid Arthritis. International Journal of Molecular Sciences. 2018; 19(1):326. https://doi.org/10.3390/ijms19010326
Chicago/Turabian StyleSzarka, Eszter, Petra Aradi, Krisztina Huber, Judit Pozsgay, Lili Végh, Anna Magyar, Gergő Gyulai, György Nagy, Bernadette Rojkovich, Éva Kiss, and et al. 2018. "Affinity Purification and Comparative Biosensor Analysis of Citrulline-Peptide-Specific Antibodies in Rheumatoid Arthritis" International Journal of Molecular Sciences 19, no. 1: 326. https://doi.org/10.3390/ijms19010326
APA StyleSzarka, E., Aradi, P., Huber, K., Pozsgay, J., Végh, L., Magyar, A., Gyulai, G., Nagy, G., Rojkovich, B., Kiss, É., Hudecz, F., & Sármay, G. (2018). Affinity Purification and Comparative Biosensor Analysis of Citrulline-Peptide-Specific Antibodies in Rheumatoid Arthritis. International Journal of Molecular Sciences, 19(1), 326. https://doi.org/10.3390/ijms19010326