2.1. Physical Properties and Microbicidal Activity of Coatings from PMMA/PDDA Dispersions
The synthesis of PMMA/PDDA NPs, described previously by Sanches et al. [
11], yielded monodisperse and cationic NPs in water dispersion named in accordance with MMA and PDDA concentrations used in the particles synthesis. For the dispersions A4, the concentrations used were 0.56 M MMA and 4 mg/mL PDDA; for A5, they were 0.56 M MMA and 5 mg/mL PDDA; and for B4, they were 1.32 M MMA and 4 mg/mL PDDA.
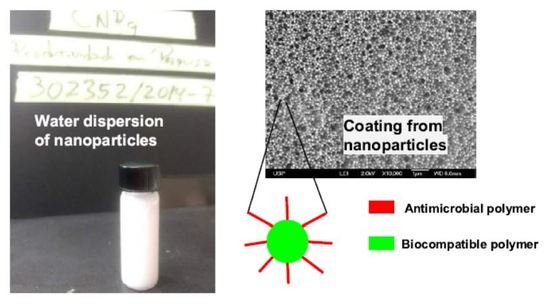
NPs in A4 have a mean diameter of 112 ± 17 nm as determined by Scanning Electron Microscopy (SEM) [
11]. Casting and drying the original A4 dispersion on silicon wafers yielded the coating shown on the SEM micrograph (
Figure 1), with the macroscopic features for the film seen on
Figure 2. The coatings were homogeneous on the hydrophilic surfaces such as the silicon wafers and the glass coverslips. However, cracks and discontinuities were visible for those on the hydrophobic polystyrene substrates (
Figure 2). The NPs structure was shown to involve a PMMA core surrounded by a PDDA shell [
11] proving that the outer cationic and hydrophilic layer clearly interacted better with the hydrophilic surfaces such as those of the silicon wafer or the glass. The coating adhesion to the hydrophilic and anionic substrates was clearly better for A5 and A4-derived coatings than for those derived from B4 (
Figure 2). The reason for this can be related to the higher relative ratio of PDDA to PMMA in A5 and A4-derived coatings than in the B4-derived ones. The interpretation for the ring appearing after casting B4 dispersion was related to the coffee-ring effect; such ring deposition occurs when liquid evaporation from the edge is replenished by liquid from the interior so that the resulting outward flow can carry most of the dispersed material to the edge [
25]. This took place for the most hydrophobic NPs, namely, those with the lowest PDDA:PMMA molar ratios represented by the B4 dispersion. Similar ring deposition pattern was also observed for hydrophobic polystyrene particles deposited on glass from a water droplet and explained from the coffee ring effect [
26]. The crack patterns visible for A4-derived coating on the polystyrene substrate were radial and similar to the ones previously described in the literature for similar systems [
27]. The poor adhesion of the hydrophilic NPs of the A4 derived-coating to the hydrophobic polystyrene sheet might also have contributed to cracks in the coating (
Figure 2).
PMMA/PDDA coatings on silicon wafers are derived from two different procedures: (1) spin-coating of lyophilized A5 in 1:1 dichloromethane: ethanol; (2) casting of A5, A4, or B4 dispersions of NPs followed by drying under vacuum.
Spin-coating allows for the preparation of lipid [
28,
29,
30] or polymer films [
22] on very smooth surfaces such as those of the silicon wafers. In the present case, the composition of a hydrophobic polymer, such as PMMA, and a hydrophilic one, such as PDDA, required a special combination of solvents in order to obtain solubilization of both in the solvents mixture.
Figure 3a–e shows the evaluation of solubilization of both polymers from the lyophilized A5 dispersion in ethanol (E):dichloromethane (D) over a range of E:D proportions. The complete solubilization only took place at 50:50% E:D. This allowed obtaining the coatings of PMMA: PDDA onto silicon wafers for evaluation of thickness, refractive index and contact angles (
Table 1). These characteristics of the hybrid films compared to those of pure PMMA coatings revealed similar thicknesses and refractive indices but higher wettability for the hybrid coatings than those determined for the pure PMMA film (
Table 1).
For coatings obtained by casting the PMMA/PDDA dispersions onto the silicon wafers, there was a consistent decrease of the contact angle upon increasing the PDDA relative amount in the dispersions from 35 ± 6 to 9 ± 2 degrees (
Table 1). Coatings obtained by casting the dispersions yielded lower contact angles than those obtained by spin-coating, reconfirming that the hydrophilic PDDA immobilized as an outer layer of the PMMA/PDDA nanoparticle imparted a more hydrophilic character to the film surface than the one of the spin-coated PMMA/PDDA (
Table 1).
The antimicrobial activity of the hybrid PMMA/PDDA coatings derived from A5, A4, and B4 casted onto glass coverslips revealed a remarkable microbicidal effect against
Escherichia coli and
Staphylococcus aureus (
Table 2). In this case, the real potency of the coatings was established over orders of magnitude by determining bacteria viability from the log of CFU/mL. Bacteria viability decreased by 10
7–10
8 colony forming units (CFU) upon interaction with the coatings for 1 h (
Table 2).
2.2. Optimization of Nanoparticles Synthesis and Conversion Percentiles
The synthesis of PMMA/PDDA NPs as dispersion A5 in absence of surfactants displayed low monomer-into-polymer conversion since only approximately 10% of the monomer mass added was converted into polymer [
11]. In order to improve conversion percentiles, the effect of monomer concentration on conversion was determined (
Figure 4;
Table 3). At 5 mg/mL PDDA, decreasing the methylmethacrylate (MMA) concentration [MMA] improved conversion, and possible reasons for this would be the relative increase in PDDA capable of stabilizing the droplet/water interface and the increased average distance between MMA droplets reducing coalescence. One should note that NPs size could also be reduced by decreasing [MMA] meaning that polymerization from smaller droplets yielded smaller NPs. At this point, stabilizing the droplet/water interface seemed crucial for improving conversion. Therefore, CTAB, DODAB, and lecithin were introduced in the reaction mixture for further stabilization of the monomer droplets.
In fact, all amphiphiles employed improved conversion (
Table 4). The most efficacious amphiphile was CTAB, followed by DODAB and lecithin. Since lecithin corresponds to a mixture of lipids and fatty acids with a net negative charge [
31,
32], at 2 mM lecithin, the NPs became negatively charged; all other NPs exhibited high and positive zeta-potentials (
Table 4). In the presence of two stabilizers (amphiphile and PDDA), conversion was substantially increased in comparison to the one in the presence of a single stabilizer. Another interesting observation refers to the lower zeta-potential for PMMA/CTAB in comparison to the one for PMMA/DODAB; this is consistent with the reported immobilization of DODAB in the PMMA polymeric matrix which is absent for CTAB, since CTAB was reported to be more mobile than DODAB easily diffusing to the outer medium from PMMA films [
22,
23]. In summary, although amphiphiles indeed improved conversion, PDDA as a second stabilizer possibly provided an additional stabilizing factor, which was the electrosteric repulsion between the MMA droplets during NP synthesis. This also represented an important stabilizing factor for the final polymeric NPs.
Figure 5 and
Table 5 show the remarkable colloidal stability of the NPs characterized by the physical properties on
Table 4. The photos taken one day and 4 months after synthesis revealed very similar macroscopic features and absence of precipitates. The analysis of sizes, polydispersities, and zeta-potentials also revealed maintenance of these physical properties of the NPs over time (
Table 5).
As compared to other similar systems in the literature, the present NPs use the self-assembly of biocompatible PMMA and the antimicrobial polymer PDDA instead of synthesizing block copolymers incorporating both functions. For example, glycosylated block copolymers were used as surfactants in butyl methacrylate emulsion polymerization [
33]. However, the antimicrobial activity was not as high as the one obtained for the coatings described in this work (
Table 2). The higher hydrophobicity inherent to the two methyl groups on the quaternary nitrogen of the PDDA molecule, as compared to the cationic glycosylated moieties, was an advantage for efficient microbicide activity. Indeed, several derivatives of PDDA evaluated for their antimicrobial activity revealed that these cationic polymers exhibit the highest activity when their chemical structure bears high frequency of hydrophobic methyl moieties [
11,
34]. The hydrophilic character of cationic antimicrobial polymers does not contribute to improvement of the antimicrobial action, although the NPs synthesis certainly benefits from their use as surfactants.
A major drawback of PMMA/PDDA NPs synthesis was the low conversion due to the relatively poor function of PDDA at the interface of MMA droplets and the surrounding water medium during NP synthesis (
Figure 4). In this work, we solved this problem by adding amphiphiles such as CTAB, DODAB, and lecithin as surfactants active as stabilizers during the NPs synthesis. In addition, we must recognize the excellent perspective of these ternary systems as antimicrobials since PDDA, DODAB, and CTAB have already being described in separate as good antimicrobial agents [
3,
8,
10,
14,
24,
35,
36,
37]. The antimicrobial properties of these ternary systems both as latexes dispersions in water and as coatings still have to be determined.