Novel Functions of Death-Associated Protein Kinases through Mitogen-Activated Protein Kinase-Related Signals
Abstract
:1. Introduction: DAPKs, MAPKs
2. Cellular Functions of DAPK Family Proteins
3. Regulation of DAPK Family Proteins
4. DAPKs in Disease
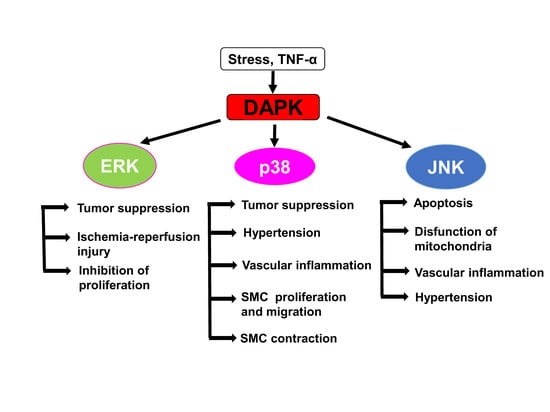
5. Cross-Talk between DAPKs and ERK Signaling
6. DAPKs as Upstream Activators of Stress-Activated Protein Kinases p38 and JNK
7. DAPKs Are Therapeutic Targets of Small Molecule Inhibitors
8. Conclusions
Funding
Conflicts of Interest
References
- Bialik, S.; Kimchi, A. The death-associated protein kinases: Structure, function, and beyond. Annu. Rev. Biochem. 2006, 75, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Cohen, O.; Feinstein, E.; Kimchi, A. Dap-kinase is a ca2+/calmodulin-dependent, cytoskeletal-associated protein kinase, with cell death-inducing functions that depend on its catalytic activity. Embo. J. 1997, 16, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Matsumoto, M.; Takeda, K.; Sanjo, H.; Akira, S. Zip kinase, a novel serine/threonine kinase which mediates apoptosis. Mol. Cell. Biol. 1998, 18, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Kogel, D.; Plottner, O.; Landsberg, G.; Christian, S.; Scheidtmann, K.H. Cloning and characterization of dlk, a novel serine/threonine kinase that is tightly associated with chromatin and phosphorylates core histones. Oncogene 1998, 17, 2645–2654. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Nomura, F.; Hoshino, K.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Akira, S. Death-associated protein kinase 2 is a new calcium/calmodulin-dependent protein kinase that signals apoptosis through its catalytic activity. Oncogene 1999, 18, 3471–3480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inbal, B.; Shani, G.; Cohen, O.; Kissil, J.L.; Kimchi, A. Death-associated protein kinase-related protein 1, a novel serine/threonine kinase involved in apoptosis. Mol. Cell. Biol. 2000, 20, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Koen, T.; Bertrand, S.; Matthias, W. Structural and functional diversity in the activity and regulation of dapk-related protein kinases. FEBS J. 2013, 280, 5533–5550. [Google Scholar]
- Detjen, K.M.; Brembeck, F.H.; Welzel, M.; Kaiser, A.; Haller, H.; Wiedenmann, B.; Rosewicz, S. Activation of protein kinase calpha inhibits growth of pancreatic cancer cells via p21(cip)-mediated g(1) arrest. J. Cell. Sci. 2000, 113, 3025–3035. [Google Scholar] [PubMed]
- Cohen, O.; Inbal, B.; Kissil, J.L.; Raveh, T.; Berissi, H.; Spivak-Kroizaman, T.; Feinstein, E.; Kimchi, A. Dap-kinase participates in tnf-alpha- and fas-induced apoptosis and its function requires the death domain. J. Cell. Biol. 1999, 146, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Sanjo, H.; Kawai, T.; Akira, S. Draks, novel serine/threonine kinases related to death-associated protein kinase that trigger apoptosis. J. Biol. Chem. 1998, 273, 29066–29071. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.-e.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (map) kinase pathways: Regulation and physiological functions*. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef] [PubMed]
- Hazzalin, C.A.; Mahadevan, L.C. Mapk-regulated transcription: A continuously variable gene switch? Nat. Rev. Mol. Cell Biol. 2002, 3, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Inbal, B.; Cohen, O.; Polak-Charcon, S.; Kopolovic, J.; Vadai, E.; Eisenbach, L.; Kimchi, A. Dap kinase links the control of apoptosis to metastasis. Nature 1997, 390, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Raveh, T.; Droguett, G.; Horwitz, M.S.; DePinho, R.A.; Kimchi, A. Dap kinase activates a p19arf/p53-mediated apoptotic checkpoint to suppress oncogenic transformation. Nat. Cell Biol 2001, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Hioki, T.; Ishii, T.; Nakajima-Iijima, S.; Uchino, S. Dap kinase activity is critical for c(2)-ceramide-induced apoptosis in pc12 cells. Eur J. Biochem 2002, 269, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.W.; Chen, C.H.; Chen, C.C.; Chen, J.Y.; Su, Y.H.; Chen, R.H. Tgf-beta induces apoptosis through smad-mediated expression of dap-kinase. Nat. Cell. Biol. 2002, 4, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Kuo, J.C.; Yao, C.C.; Chen, R.H. Dap-kinase induces apoptosis by suppressing integrin activity and disrupting matrix survival signals. J. Cell. Biol. 2002, 159, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Bialik, S.; Kimchi, A. Lethal weapons: Dap-kinase, autophagy and cell death: Dap-kinase regulates autophagy. Curr. Opin. Cell. Biol. 2010, 22, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Levin-Salomon, V.; Bialik, S.; Kimchi, A. Dap-kinase and autophagy. Apoptosis 2014, 19, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Gade, P.; Roy, S.K.; Li, H.; Nallar, S.C.; Kalvakolanu, D.V. Critical role for transcription factor c/ebp-beta in regulating the expression of death-associated protein kinase 1. Mol. Cell. Biol. 2008, 28, 2528–2548. [Google Scholar] [CrossRef] [PubMed]
- Gozuacik, D.; Bialik, S.; Raveh, T.; Mitou, G.; Shohat, G.; Sabanay, H.; Mizushima, N.; Yoshimori, T.; Kimchi, A. Dap-kinase is a mediator of endoplasmic reticulum stress-induced caspase activation and autophagic cell death. Cell Death Differ. 2008, 15, 1875–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geering, B.; Stoeckle, C.; Rozman, S.; Oberson, K.; Benarafa, C.; Simon, H.U. Dapk2 positively regulates motility of neutrophils and eosinophils in response to intermediary chemoattractants. J. Leukoc Biol. 2014, 95, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Menon, M.; Zhang, D.; Torbett, B.; Oxburgh, L.; Tschan, M.; Houde, E.; Wojchowski, D.M. Attenuation of epo-dependent erythroblast formation by death-associated protein kinase-2. Blood 2008, 112, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Okada, M.; Yamawaki, H. Zipper interacting protein kinase (zipk): Function and signaling. Apoptosis 2014, 19, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Van Eldik, L.J. Structure and enzymology of a death-associated protein kinase. Trends Pharmacol. Sci. 2002, 23, 302–304. [Google Scholar] [CrossRef]
- Okamoto, M.; Takayama, K.; Shimizu, T.; Muroya, A.; Furuya, T. Structure-activity relationship of novel dapk inhibitors identified by structure-based virtual screening. Bioorg. Med. Chem. 2010, 18, 2728–2734. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Ray, P.S.; Arif, A.; Brady, A.K.; Kinter, M.; Fox, P.L. Dapk-zipk-l13a axis constitutes a negative-feedback module regulating inflammatory gene expression. Mol. Cell. 2008, 32, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Pelled, D.; Raveh, T.; Riebeling, C.; Fridkin, M.; Berissi, H.; Futerman, A.H.; Kimchi, A. Death-associated protein (dap) kinase plays a central role in ceramide-induced apoptosis in cultured hippocampal neurons. J. Biol. Chem. 2002, 277, 1957–1961. [Google Scholar] [CrossRef] [PubMed]
- Martoriati, A.; Doumont, G.; Alcalay, M.; Bellefroid, E.; Pelicci, P.G.; Marine, J.C. Dapk1, encoding an activator of a p19arf-p53-mediated apoptotic checkpoint, is a transcription target of p53. Oncogene 2005, 24, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Chawla-Sarkar, M.; Lindner, D.J.; Liu, Y.F.; Williams, B.R.; Sen, G.C.; Silverman, R.H.; Borden, E.C. Apoptosis and interferons: Role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 2003, 8, 237–249. [Google Scholar] [CrossRef] [PubMed]
- de Diego, I.; Kuper, J.; Bakalova, N.; Kursula, P.; Wilmanns, M. Molecular basis of the death-associated protein kinase-calcium/calmodulin regulator complex. Sci. Signal. 2010, 3, ra6. [Google Scholar] [CrossRef] [PubMed]
- Shani, G.; Henis-Korenblit, S.; Jona, G.; Gileadi, O.; Eisenstein, M.; Ziv, T.; Admon, A.; Kimchi, A. Autophosphorylation restrains the apoptotic activity of drp-1 kinase by controlling dimerization and calmodulin binding. Embo. J. 2001, 20, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Shohat, G.; Spivak-Kroizman, T.; Cohen, O.; Bialik, S.; Shani, G.; Berrisi, H.; Eisenstein, M.; Kimchi, A. The pro-apoptotic function of death-associated protein kinase is controlled by a unique inhibitory autophosphorylation-based mechanism. J. Biol. Chem. 2001, 276, 47460–47467. [Google Scholar] [CrossRef] [PubMed]
- Shang, T.; Joseph, J.; Hillard, C.J.; Kalyanaraman, B. Death-associated protein kinase as a sensor of mitochondrial membrane potential: Role of lysosome in mitochondrial toxin-induced cell death. J. Biol. Chem. 2005, 280, 34644–34653. [Google Scholar] [CrossRef] [PubMed]
- Inbal, B.; Bialik, S.; Sabanay, I.; Shani, G.; Kimchi, A. Dap kinase and drp-1 mediate membrane blebbing and the formation of autophagic vesicles during programmed cell death. J. Cell. Biol. 2002, 157, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Isshiki, K.; Matsuda, S.; Tsuji, A.; Yuasa, K. Cgmp-dependent protein kinase i promotes cell apoptosis through hyperactivation of death-associated protein kinase 2. Biochem. Biophys. Res. Commun. 2012, 422, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Shani, G.; Marash, L.; Gozuacik, D.; Bialik, S.; Teitelbaum, L.; Shohat, G.; Kimchi, A. Death-associated protein kinase phosphorylates zip kinase, forming a unique kinase hierarchy to activate its cell death functions. Mol. Cell. Biol. 2004, 24, 8611–8626. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kelnar, K.; Liu, B.; Chen, X.; Calhoun-Davis, T.; Li, H.; Patrawala, L.; Yan, H.; Jeter, C.; Honorio, S.; et al. The microrna mir-34a inhibits prostate cancer stem cells and metastasis by directly repressing cd44. Nat. Med. 2011, 17, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Kondo, H. Molecular cloning and developmental expression of a rat homologue of death-associated protein kinase in the nervous system. Mol. Brain Res. 1997, 52, 249–256. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takahashi, H.; Nakamura, T.; Hioki, T.; Nagayama, S.; Ooashi, N.; Sun, X.; Ishii, T.; Kudo, Y.; Nakajima-Iijima, S.; et al. Developmental changes in distribution of death-associated protein kinase mrnas. J. Neurosci. Res. 1999, 58, 674–683. [Google Scholar] [CrossRef]
- Velentza, A.V.; Wainwright, M.S.; Zasadzki, M.; Mirzoeva, S.; Schumacher, A.M.; Haiech, J.; Focia, P.J.; Egli, M.; Watterson, D.M. An aminopyridazine-based inhibitor of a pro-apoptotic protein kinase attenuates hypoxia-ischemia induced acute brain injury. Bioorg. Med. Chem. Lett. 2003, 13, 3465–3470. [Google Scholar] [CrossRef]
- Schumacher, A.M.; Velentza, A.V.; Watterson, D.M.; Wainwright, M.S. Dapk catalytic activity in the hippocampus increases during the recovery phase in an animal model of brain hypoxic-ischemic injury. Biochim. Biophys. Acta 2002, 1600, 128–137. [Google Scholar] [CrossRef]
- Henshall, D.C.; Araki, T.; Schindler, C.K.; Shinoda, S.; Lan, J.Q.; Simon, R.P. Expression of death-associated protein kinase and recruitment to the tumor necrosis factor signaling pathway following brief seizures. J. Neurochem. 2003, 86, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Shinoda, S.; Schindler, C.K.; Quan-Lan, J.; Meller, R.; Taki, W.; Simon, R.P.; Henshall, D.C. Expression, interaction, and proteolysis of death-associated protein kinase and p53 within vulnerable and resistant hippocampal subfields following seizures. Hippocampus 2004, 14, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Mor, I.; Carlessi, R.; Ast, T.; Feinstein, E.; Kimchi, A. Death-associated protein kinase increases glycolytic rate through binding and activation of pyruvate kinase. Oncogene 2012, 31, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Grupe, A.; Rowland, C.; Nowotny, P.; Kauwe, J.S.; Smemo, S.; Hinrichs, A.; Tacey, K.; Toombs, T.A.; Kwok, S.; et al. Dapk1 variants are associated with Alzheimer’s disease and allele-specific expression. Hum. Mol. Genet. 2006, 15, 2560–2568. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Grupe, A.; Rowland, C.; Nowotny, P.; Kauwe, J.S.K.; Smemo, S.; Hinrichs, A.; Tacey, K.; Toombs, T.A.; Kwok, S.; et al. Dapk1 variants are associated with Alzheimer’s disease and allele-specific expression. Hum. Mol. Genet. 2006, 15, 2560–2568. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Xu, X.; Peng, L.; Zhong, X.; Zhang, W.; Soundarapandian, M.M.; Balel, C.; Wang, M.; Jia, N.; Zhang, W.; et al. Dapk1 interaction with nmda receptor nr2b subunits mediates brain damage in stroke. Cell 2010, 140, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, M.; Soriano, L.; Wieloch, T.; Nikolich, K.; Urfer, R.; Oksenberg, D. Death-associated protein kinase is activated by dephosphorylation in response to cerebral ischemia. J. Biol. Chem. 2005, 280, 42290–42299. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.M.; Balin, B.J.; Otvos, L., Jr.; Trojanowski, J.Q. A68: A major subunit of paired helical filaments and derivatized forms of normal tau. Science 1991, 251, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Spillantini, M.G.; Cairns, N.J.; Crowther, R.A. Tau proteins of alzheimer paired helical filaments: Abnormal phosphorylation of all six brain isoforms. Neuron 1992, 8, 159–168. [Google Scholar] [CrossRef]
- Wu, P.R.; Tsai, P.I.; Chen, G.C.; Chou, H.J.; Huang, Y.P.; Chen, Y.H.; Lin, M.Y.; Kimchi, A.; Chien, C.T.; Chen, R.H. Dapk activates mark1/2 to regulate microtubule assembly, neuronal differentiation, and tau toxicity. Cell Death Differ. 2011, 18, 1507–1520. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; You, M.H.; Chen, C.H.; Lee, S.; Hong, Y.; Hong, Y.; Kimchi, A.; Zhou, X.Z.; Lee, T.H. Death-associated protein kinase 1 has a critical role in aberrant tau protein regulation and function. Cell Death Dis. 2014, 5, e1237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, H.G.; Wang, Y.T. Blocking the deadly effects of the nmda receptor in stroke. Cell 2010, 140, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Cohen, O.; Kimchi, A. Dap-kinase: From functional gene cloning to establishment of its role in apoptosis and cancer. Cell Death Differ. 2001, 8, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Bai, T.; Toujima, S.; Utsunomiya, T.; Utsunomiya, H.; Yukawa, K.; Tanaka, J. Impaired death-associated protein kinase-mediated survival signals in 5-fluorouracil-resistant human endometrial adenocarcinoma cells. Oncol. Rep. 2012, 28, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Liggett, T.E.; Melnikov, A.A.; Monitto, C.L.; Kusuke, D.; Shiga, K.; Kobayashi, T.; Horii, A.; Chatterjee, A.; Levenson, V.V.; et al. Methylation of death-associated protein kinase is associated with cetuximab and erlotinib resistance. Cell Cycle 2012, 11, 1656–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Q.; Chen, Y.; Wu, Y. Enhancing apoptosis and overcoming resistance of gemcitabine in pancreatic cancer with bortezomib: A role of death-associated protein kinase-related apoptosis-inducing protein kinase 1. Tumori J. 2009, 95, 796–803. [Google Scholar] [CrossRef]
- Bai, T.; Tanaka, T.; Yukawa, K.; Umesaki, N. A novel mechanism for acquired cisplatin-resistance: Suppressed translation of death-associated protein kinase mrna is insensitive to 5-aza-2′-deoxycitidine and trichostatin in cisplatin-resistant cervical squamous cancer cells. Int. J. Oncol. 2006, 28, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, M.-M.; Shu, H.-B.; Li, S. Death-associated protein kinase 1 is an irf3/7-interacting protein that is involved in the cellular antiviral immune response. Cell. Mol. Immunol. 2014, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- McGargill, M.A.; Wen, B.G.; Walsh, C.M.; Hedrick, S.M. A deficiency in drak2 results in a t cell hypersensitivity and an unexpected resistance to autoimmunity. Immunity 2004, 21, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hooshdaran, B.; Rafiq, K.; Xi, H.; Kolpakov, M.; Liu, S.; Zhang, X.; Chen, X.; Houser, S.R.; Koch, W.J.; et al. Abstract 16144: Death associated protein kinase mediates myofibril degeneration and myocyte apoptosis induced by beta-adrenergic receptors. Circulation 2016, 134, A16144. [Google Scholar]
- Chuang, Y.-T.; Lin, Y.-C.; Lin, K.-H.; Chou, T.-F.; Kuo, W.-C.; Yang, K.-T.; Wu, P.-R.; Chen, R.-H.; Kimchi, A.; Lai, M.-Z. Tumor suppressor death-associated protein kinase is required for full IL-1beta production. Blood 2011, 117, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.T.; Fang, L.W.; Lin-Feng, M.H.; Chen, R.H.; Lai, M.Z. The tumor suppressor death-associated protein kinase targets to tcr-stimulated nf-kappa b activation. J. Immunol. 2008, 180, 3238–3249. [Google Scholar] [CrossRef] [PubMed]
- Nakav, S.; Cohen, S.; Feigelson, S.W.; Bialik, S.; Shoseyov, D.; Kimchi, A.; Alon, R. Tumor suppressor death-associated protein kinase attenuates inflammatory responses in the lung. Am. J. Respir. Cell. Mol. Biol. 2012, 46, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Kuester, D.; Guenther, T.; Biesold, S.; Hartmann, A.; Bataille, F.; Ruemmele, P.; Peters, B.; Meyer, F.; Schubert, D.; Bohr, U.R.; et al. Aberrant methylation of dapk in long-standing ulcerative colitis and ulcerative colitis-associated carcinoma. Pathol. Res. Pract. 2010, 206, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Martinet, W.; Schrijvers, D.M.; De Meyer, G.R.; Thielemans, J.; Knaapen, M.W.; Herman, A.G.; Kockx, M.M. Gene expression profiling of apoptosis-related genes in human atherosclerosis: Upregulation of death-associated protein kinase. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Rennier, K.; Ji, J.Y. Shear stress regulates expression of death-associated protein kinase in suppressing tnfalpha-induced endothelial apoptosis. J. Cell. Physiol 2012, 227, 2398–2411. [Google Scholar] [CrossRef] [PubMed]
- Rennier, K.; Ji, J.Y. Effect of shear stress and substrate on endothelial dapk expression, caspase activity, and apoptosis. BMC Res. Notes 2013, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, M.; Tschan, M.P.; Britschgi, C.; Britschgi, A.; Hugli, B.; Grob, T.J.; Leupin, N.; Mueller, B.U.; Simon, H.U.; Ziemiecki, A.; et al. The death-associated protein kinase 2 is up-regulated during normal myeloid differentiation and enhances neutrophil maturation in myeloid leukemic cells. J. Leukoc. Biol. 2007, 81, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Guay, J.A.; Wojchowski, D.M.; Fang, J.; Oxburgh, L. Death associated protein kinase 2 is expressed in cortical interstitial cells of the mouse kidney. BMC Res. Notes 2014, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Brognard, J.; Zhang, Y.W.; Puto, L.A.; Hunter, T. Cancer-associated loss-of-function mutations implicate dapk3 as a tumor-suppressing kinase. Cancer Res. 2011, 71, 3152–3161. [Google Scholar] [CrossRef] [PubMed]
- Leister, P.; Felten, A.; Chasan, A.I.; Scheidtmann, K.H. Zip kinase plays a crucial role in androgen receptor-mediated transcription. Oncogene 2008, 27, 3292–3300. [Google Scholar] [CrossRef] [PubMed]
- Togi, S.; Ikeda, O.; Kamitani, S.; Nakasuji, M.; Sekine, Y.; Muromoto, R.; Nanbo, A.; Oritani, K.; Kawai, T.; Akira, S.; et al. Zipper-interacting protein kinase (zipk) modulates canonical wnt/beta-catenin signaling through interaction with nemo-like kinase and t-cell factor 4 (nlk/tcf4). J. Biol. Chem. 2011, 286, 19170–19177. [Google Scholar] [CrossRef] [PubMed]
- Kake, S.; Usui, T.; Ohama, T.; Yamawaki, H.; Sato, K. Death-associated protein kinase 3 controls the tumor progression of a549 cells through erk mapk/c-myc signaling. Oncol. Rep. 2017, 37, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Okada, M.; Hara, Y.; Yamawaki, H. Exploring calmodulin-related proteins, which mediate development of hypertension, in vascular tissues of spontaneous hypertensive rats. Biochemical. Biophysical. Res. Commun. 2011, 405, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Okada, M.; Hara, Y.; Yamawaki, H. Death-associated protein kinase 3 mediates vascular inflammation and development of hypertension in spontaneously hypertensive rats. Hypertension 2012, 60, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.E.; Ahn, D.S.; Morgan, K.G.; Lee, Y.H. Enhanced contractility and myosin phosphorylation induced by ca(2+)-independent mlck activity in hypertensive rats. Cardiovasc. Res. 2011, 91, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Usui, T.; Sakatsume, T.; Nijima, R.; Otani, K.; Kazama, K.; Morita, T.; Kameshima, S.; Okada, M.; Yamawaki, H. Death-associated protein kinase 3 mediates vascular structural remodelling via stimulating smooth muscle cell proliferation and migration. Clin. Sci. 2014, 127, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Ihara, E.; MacDonald, J.A. The regulation of smooth muscle contractility by zipper-interacting protein kinase. Can. J. Physiol. Pharmacol. 2007, 85, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of atp-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Sebolt-Leopold, J.S.; Herrera, R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat. Rev. Cancer 2004, 4, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.S.; Afanasiev, S.; Aidala, C.; Ajitanand, N.N.; Akiba, Y.; Alexander, J.; Amirikas, R.; Aphecetche, L.; Aronson, S.H.; Averbeck, R.; et al. Measurement of transverse single-spin asymmetries for midrapidity production of neutral pions and charged hadrons in polarized p + p collisions at square root(s) = 200 gev. Phys. Rev. Lett. 2005, 95, 202001. [Google Scholar] [CrossRef] [PubMed]
- Ballif, B.A.; Blenis, J. Molecular mechanisms mediating mammalian mitogen-activated protein kinase (mapk) kinase (mek)-mapk cell survival signals. Cell Growth Differ. 2001, 12, 397–408. [Google Scholar] [PubMed]
- Stevens, C.; Lin, Y.; Sanchez, M.; Amin, E.; Copson, E.; White, H.; Durston, V.; Eccles, D.M.; Hupp, T. A germ line mutation in the death domain of dapk-1 inactivates erk-induced apoptosis. J. Biol. Chem. 2007, 282, 13791–13803. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, M.L.; Ramana, C.V.; Hamilton, M.; Taylor, W.R.; DePrimo, S.E.; Bean, L.J.; Agarwal, A.; Agarwal, M.K.; Wolfman, A.; Stark, G.R. Regulation of p53 expression by the ras-map kinase pathway. Oncogene 2001, 20, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Wu, Y.; Xian, W.; Song, L.; Hu, L.; Pan, S.; Liu, M.; Yao, S.; Pei, L.; Shang, Y. Dapk1-erk signal mediates oxygen glucose deprivation reperfusion induced apoptosis in mouse n2a cells. J. Neurol. Sc.i 2018, 387, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.M.; Johnson, G.L. Fibroblast growth factor-2 suppression of tumor necrosis factor alpha-mediated apoptosis requires ras and the activation of mitogen-activated protein kinase. J. Biol. Chem. 1996, 271, 14560–14566. [Google Scholar] [CrossRef] [PubMed]
- Kayali, A.G.; Austin, D.A.; Webster, N.J. Stimulation of mapk cascades by insulin and osmotic shock: Lack of an involvement of p38 mitogen-activated protein kinase in glucose transport in 3t3-l1 adipocytes. Diabetes 2000, 49, 1783–1793. [Google Scholar] [CrossRef] [PubMed]
- Puls, A.; Eliopoulos, A.G.; Nobes, C.D.; Bridges, T.; Young, L.S.; Hall, A. Activation of the small gtpase cdc42 by the inflammatory cytokines tnf(alpha) and il-1, and by the epstein-barr virus transforming protein lmp1. J. Cell Sci. 1999, 112, 2983–2992. [Google Scholar] [PubMed]
- Fritz, G.; Kaina, B. Activation of c-jun n-terminal kinase 1 by uv irradiation is inhibited by wortmannin without affecting c-iun expression. Mol. Cell. Biol. 1999, 19, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Wagner, E.F.; Nebreda, A.R. Signal integration by jnk and p38 mapk pathways in cancer development. Nat. Rev. Cancer 2009, 9, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Bajbouj, K.; Poehlmann, A.; Kuester, D.; Drewes, T.; Haase, K.; Hartig, R.; Teller, A.; Kliche, S.; Walluscheck, D.; Ivanovska, J.; et al. Retracted: Identification of phosphorylated p38 as a novel dapk-interacting partner during tnfalpha-induced apoptosis in colorectal tumor cells. Am. J. Pathol. 2009, 175, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Sakon, S.; Xue, X.; Takekawa, M.; Sasazuki, T.; Okazaki, T.; Kojima, Y.; Piao, J.H.; Yagita, H.; Okumura, K.; Doi, T.; et al. Nf-kappab inhibits tnf-induced accumulation of ros that mediate prolonged mapk activation and necrotic cell death. Embo J. 2003, 22, 3898–3909. [Google Scholar] [CrossRef] [PubMed]
- Kamata, H.; Honda, S.; Maeda, S.; Chang, L.; Hirata, H.; Karin, M. Reactive oxygen species promote tnfalpha-induced death and sustained jnk activation by inhibiting map kinase phosphatases. Cell 2005, 120, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg-Lerner, A.; Kimchi, A. Dap kinase regulates jnk signaling by binding and activating protein kinase d under oxidative stress. Cell Death Differ. 2007, 14, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, C.R.; Georgiou, M.L.; Misterek, M.B.; Stocker, S.; Chater, E.R.; Munro, C.E.; Pardo, O.E.; Seckl, M.J.; Costa-Pereira, A.P. Dapk2 regulates oxidative stress in cancer cells by preserving mitochondrial function. Cell Death Dis 2015, 6, e1671. [Google Scholar] [CrossRef] [PubMed]
- Watterson, D.M.; Mirzoeva, S.; Guo, L.; Whyte, A.; Bourguignon, J.J.; Hilbert, M.; Haiech, J.; Van Eldik, L.J. Ligand modulation of glial activation: Cell permeable, small molecule inhibitors of serine-threonine protein kinases can block induction of interleukin 1 beta and nitric oxide synthase ii. Neurochem. Int. 2001, 39, 459–468. [Google Scholar] [CrossRef]
- Mirzoeva, S.; Sawkar, A.; Zasadzki, M.; Guo, L.; Velentza, A.V.; Dunlap, V.; Bourguignon, J.-J.; Ramstrom, H.; Haiech, J.; Van Eldik, L.J.; et al. Discovery of a 3-amino-6-phenyl-pyridazine derivative as a new synthetic antineuroinflammatory compound. J. Med. Chem. 2002, 45, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Takayama, K.; Shimizu, T.; Ishida, K.; Takahashi, O.; Furuya, T. Identification of death-associated protein kinases inhibitors using structure-based virtual screening. J. Mèd. Chem. 2009, 52, 7323–7327. [Google Scholar] [CrossRef] [PubMed]
- Gandesiri, M.; Chakilam, S.; Ivanovska, J.; Benderska, N.; Ocker, M.; Di Fazio, P.; Feoktistova, M.; Gali-Muhtasib, H.; Rave-Frank, M.; Prante, O.; et al. Dapk plays an important role in panobinostat-induced autophagy and commits cells to apoptosis under autophagy deficient conditions. Apoptosis 2012, 17, 1300–1315. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Han, Y.; Xu, L.Z.; Yuan, K.; Zhang, R.X.; Sun, C.Y.; Xu, D.F.; Yuan, M.; Deng, J.H.; Meng, S.Q.; et al. Uncoupling dapk1 from nmda receptor glun2b subunit exerts rapid antidepressant-like effects. Mol. Psychiatry 2018, 23, 597–608. [Google Scholar] [CrossRef] [PubMed]
- de las Infantas, M.J.P.; Torres-Rusillo, S.; Unciti-Broceta, J.D.; Fernandez-Rubio, P.; Luque-Gonzalez, M.A.; Gallo, M.A.; Unciti-Broceta, A.; Molina, I.J.; Diaz-Mochon, J.J. Synthesis of 6,8,9 poly-substituted purine analogue libraries as pro-apoptotic inducers of human leukemic lymphocytes and dapk-1 inhibitors. Org. Biomol. Chem. 2015, 13, 5224–5234. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.D.; Chen, L.; Guo, L.B.; Hupp, T.R.; Lin, Y. Evaluating dapk as a therapeutic target. Apoptosis 2014, 19, 371–386. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.Y.; Lee, S.J.; Nam, K.W.; Shin, J.; Oh, K.B.; Kim, K.H.; Mar, W. Amelioration of oxygen and glucose deprivation-induced neuronal death by chloroform fraction of bay leaves (Laurus nobilis). Biosci. Biotechnol. Biochem. 2010, 74, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.A.; Singer, M.R.; Sutherland, C.; Redondo, C.; Alexander, L.T.; Hughes, P.F.; Knapp, S.; Gurley, S.B.; Sparks, M.A.; MacDonald, J.A.; et al. Targeting pim kinases and dapk3 to control hypertension. Cell Chem. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, J.A.; Sutherland, C.; Carlson, D.A.; Bhaidani, S.; Al-Ghabkari, A.; Sward, K.; Haystead, T.A.; Walsh, M.P. A small molecule pyrazolo [3,4-d]pyrimidinone inhibitor of zipper-interacting protein kinase suppresses calcium sensitization of vascular smooth muscle. Mol. Pharmacol. 2016, 89, 105–117. [Google Scholar] [CrossRef] [PubMed]





| Name | Structure | Types of Inhibitors | IC50 |
|---|---|---|---|
| Alkylated 3-amino-6-phenylpyridazine |  | ATP-competitive | 13, 22 mM for DAPK1 |
| (4Z)-4-(3-pyridylmethylene)-2-styryl-oxazol-5-one |  | ATP-competitive | 69 and 225 nM against DAPK1 and DAPK3, respectively |
| (4Z)-2-[(E)-2-Phenylethenyl)-4-(3-pyridinylmethylene)-5(4H)-oxazolone (TC-DAPK6) |  | ATP-competitive | 69 and 225 nM against DAPK1 and DAPK3, respectively |
| 6-benzyloxy-9-tert-butyl-8-phenyl-9H-purine (6d) |  | ATP-competitive | 2.5 mM for DAPK1 |
| HS56 |  | ATP-competitive | 1 mM for DAPK3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elbadawy, M.; Usui, T.; Yamawaki, H.; Sasaki, K. Novel Functions of Death-Associated Protein Kinases through Mitogen-Activated Protein Kinase-Related Signals. Int. J. Mol. Sci. 2018, 19, 3031. https://doi.org/10.3390/ijms19103031
Elbadawy M, Usui T, Yamawaki H, Sasaki K. Novel Functions of Death-Associated Protein Kinases through Mitogen-Activated Protein Kinase-Related Signals. International Journal of Molecular Sciences. 2018; 19(10):3031. https://doi.org/10.3390/ijms19103031
Chicago/Turabian StyleElbadawy, Mohamed, Tatsuya Usui, Hideyuki Yamawaki, and Kazuaki Sasaki. 2018. "Novel Functions of Death-Associated Protein Kinases through Mitogen-Activated Protein Kinase-Related Signals" International Journal of Molecular Sciences 19, no. 10: 3031. https://doi.org/10.3390/ijms19103031
APA StyleElbadawy, M., Usui, T., Yamawaki, H., & Sasaki, K. (2018). Novel Functions of Death-Associated Protein Kinases through Mitogen-Activated Protein Kinase-Related Signals. International Journal of Molecular Sciences, 19(10), 3031. https://doi.org/10.3390/ijms19103031