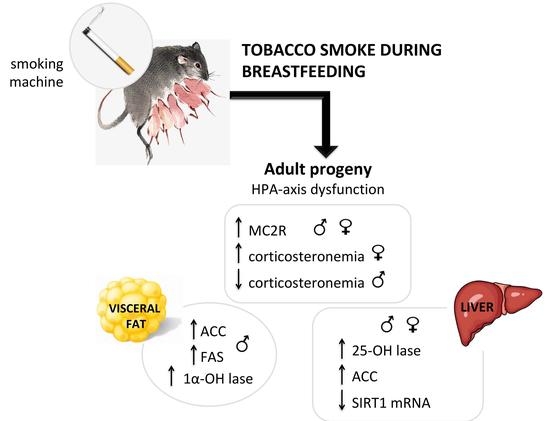
Cigarette Smoke During Breastfeeding in Rats Changes Glucocorticoid and Vitamin D Status in Obese Adult Offspring
Abstract
:1. Introduction
2. Results
2.1. Histology and Lipid Content
2.2. Serum Corticosterone and Adrenal ACTH Receptor (MC2R).
2.3. Glucocorticoid Metabolism and Action
2.4. Tissue Metabolism and Serum Levels of 25 (OH) D
2.5. Lipogenesis in Visceral Adipose Tissue
2.6. Lipogenesis in Liver
3. Discussion
4. Materials and Methods
4.1. Animal Research
Experimental Model of Direct Tobacco Smoke Exposure during Suckling Period
4.2. Histology
4.3. Hepatic Determination of Triglyceride and Cholesterol Contents
4.4. Serum Hormones Analysis—Radioimmunoassay (RIA) and Electrochemiluminescence Immunoassay (ECLIA)
4.5. Western Blotting
4.6. Sirtuin 1 (Sirt1) mRNA Expression in the Liver
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflict of Interest
References
- Taghizadeh, N.; Vonk, J.M.; Boezen, H.M. Lifetime Smoking History and Cause-Specific Mortality in a Cohort Study with 43 Years of Follow-Up. PLoS ONE 2016, 11, e0153310. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, N.; de Almeida, L.M.; Szklo, M.; Figueiredo, V.C.; Szklo, A.S. Assessing the relationship between smoking and abdominal obesity in a National Survey of Adolescents in Brazil. Prev. Med. 2018, 111, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Van Minh, H.; Giang, K.B.; Ngoc, N.B.; Hai, P.T.; Huyen, D.T.; Khue, L.N.; Lam, N.T.; Nga, P.T.; Quan, N.T.; Xuyen, N.T. Prevalence of tobacco smoking in Vietnam: Findings from the Global Adult Tobacco Survey 2015. Int. J. Public Health 2017, 62, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The developmental origins of adult disease. J. Am. Coll. Nutr. 2004, 23, 588S–595S. [Google Scholar] [CrossRef] [PubMed]
- Vik, T.; Jacobsen, G.; Vatten, L.; Bakketeig, L.S. Pre- and post-natal growth in children of women who smoked in pregnancy. Early Hum. Dev. 1996, 45, 245–255. [Google Scholar] [CrossRef]
- Bronars, C.; Patten, C.; Koller, K.; Hatsukami, D.; Flanagan, C.A.; Decker, P.A.; Hanson, A.; Wolfe, A.; Hughes, C.; Benowitz, N.; et al. Perceived risks and reasons to smoke cigarettes during pregnancy among Alaska native women. Ethn Health 2018, 23, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Rydell, M.; Magnusson, C.; Cnattingius, S.; Granath, F.; Svensson, A.C.; Galanti, M.R. Exposure to maternal smoking during pregnancy as a risk factor for tobacco use in adult offspring. Am. J. Epidemiol. 2014, 179, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Giglia, R.C.; Binns, C.W.; Alfonso, H.S.; Zhao, Y. Which mothers smoke before, during and after pregnancy? Public Health 2007, 121, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Napierala, M.; Mazela, J.; Merritt, T.A.; Florek, E. Tobacco smoking and breastfeeding: Effect on the lactation process, breast milk composition and infant development. A critical review. Environ. Res. 2016, 151, 321–338. [Google Scholar] [CrossRef] [PubMed]
- De Moura, E.G.; Lisboa, P.C.; Passos, M.C. Neonatal programming of neuroimmunomodulation--role of adipocytokines and neuropeptides. Neuroimmunomodulation 2008, 15, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Muniesa, P.; Martinez-Gonzalez, M.A.; Hu, F.B.; Despres, J.P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat. Rev. Dis. Primers 2017, 3, 17034. [Google Scholar] [CrossRef] [PubMed]
- Von Kries, R.; Toschke, A.M.; Wurmser, H.; Sauerwald, T.; Koletzko, B. Reduced risk for overweight and obesity in 5- and 6-y-old children by duration of sleep—A cross-sectional study. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Durmus, B.; Ay, L.; Hokken-Koelega, A.C.; Raat, H.; Hofman, A.; Steegers, E.A.; Jaddoe, V.W. Maternal smoking during pregnancy and subcutaneous fat mass in early childhood. The Generation R Study. Eur. J. Epidemiol. 2011, 26, 295–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wideroe, M.; Vik, T.; Jacobsen, G.; Bakketeig, L.S. Does maternal smoking during pregnancy cause childhood overweight? Paediatr. Perinat. Epidemiol. 2003, 17, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Santos-Silva, A.P.; Oliveira, E.; Pinheiro, C.R.; Santana, A.C.; Nascimento-Saba, C.C.; Abreu-Villaca, Y.; Moura, E.G.; Lisboa, P.C. Endocrine effects of tobacco smoke exposure during lactation in weaned and adult male offspring. J. Endocrinol. 2013, 218, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisboa, P.C.; Soares, P.N.; Peixoto, T.C.; Carvalho, J.C.; Calvino, C.; Rodrigues, V.S.T.; Bernardino, D.N.; Younes-Rapozo, V.; Manhaes, A.C.; de Oliveira, E.; et al. Effects of cigarette smoke exposure during suckling on food intake, fat mass, hormones, and biochemical profile of young and adult female rats. Endocrine 2017, 57, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Strissel, K.J.; Stancheva, Z.; Miyoshi, H.; Perfield, J.W., 2nd; DeFuria, J.; Jick, Z.; Greenberg, A.S.; Obin, M.S. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 2007, 56, 2910–2918. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.N.; Hua, S.Y.; Chiu, C.T.; Li, C.Y. Maternal Exposure to Air Pollutants and Risk of Gestational Diabetes Mellitus in Taiwan. Int. J. Environ. Res. Public Health 2017, 14, 1604. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, J.; DeLuca, H.F. Where is the vitamin D receptor? Arch. Biochem. Biophys. 2012, 523, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Bae, S.; Yoon, Y. Anti-adipogenic effects of 1,25-dihydroxyvitamin D3 are mediated by the maintenance of the wingless-type MMTV integration site/beta-catenin pathway. Int. J. Mol. Med. 2012, 30, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- John, K.; Marino, J.S.; Sanchez, E.R.; Hinds, T.D., Jr. The glucocorticoid receptor: Cause of or cure for obesity? Am. J. Physiol. Endocrinol. Metab. 2016, 310, E249–E257. [Google Scholar] [CrossRef] [PubMed]
- Manolopoulos, K.; Thornhill, H.; Thomas, J.; Arlt, W.; Tomlinson, J. Association between hypercortisolaemia and adipose tissue blood flow in vivo. Lancet 2015, 385 (Suppl. 1), S63. [Google Scholar] [CrossRef]
- Yu, J.; Yu, B.; He, J.; Zheng, P.; Mao, X.; Han, G.; Chen, D. Chronic glucocorticoid exposure-induced epididymal adiposity is associated with mitochondrial dysfunction in white adipose tissue of male C57BL/6J mice. PLoS ONE 2014, 9, e112628. [Google Scholar] [CrossRef] [PubMed]
- De Guia, R.M.; Herzig, S. How Do Glucocorticoids Regulate Lipid Metabolism? Adv. Exp. Med. Biol. 2015, 872, 127–144. [Google Scholar] [PubMed]
- Masuzaki, H.; Paterson, J.; Shinyama, H.; Morton, N.M.; Mullins, J.J.; Seckl, J.R.; Flier, J.S. A transgenic model of visceral obesity and the metabolic syndrome. Science 2001, 294, 2166–2170. [Google Scholar] [CrossRef] [PubMed]
- Ghali, O.; Broux, O.; Falgayrac, G.; Haren, N.; van Leeuwen, J.P.; Penel, G.; Hardouin, P.; Chauveau, C. Dexamethasone in osteogenic medium strongly induces adipocyte differentiation of mouse bone marrow stromal cells and increases osteoblast differentiation. BMC Cell Biol. 2015, 16, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockall, A.G.; Sohaib, S.A.; Evans, D.; Kaltsas, G.; Isidori, A.M.; Monson, J.P.; Besser, G.M.; Grossman, A.B.; Reznek, R.H. Hepatic steatosis in Cushing’s syndrome: A radiological assessment using computed tomography. Eur J. Endocrinol 2003, 149, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Ferrau, F.; Korbonits, M. Metabolic comorbidities in Cushing’s syndrome. Eur. J. Endocrinol. 2015, 173, M133–M157. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Norman, A.W.; Okamura, W.H.; Sen, A.; Zemel, M.B. 1alpha,25-Dihydroxyvitamin D3 modulates human adipocyte metabolism via nongenomic action. FASEB J. 2001, 15, 2751–2753. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, J.M.; Tzameli, I.; Astapova, I.; Lam, F.S.; Flier, J.S.; Hollenberg, A.N. Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3-L1 cells. J. Biol. Chem. 2006, 281, 11205–11213. [Google Scholar] [CrossRef] [PubMed]
- Ross, S.E.; Hemati, N.; Longo, K.A.; Bennett, C.N.; Lucas, P.C.; Erickson, R.L.; MacDougald, O.A. Inhibition of adipogenesis by Wnt signaling. Science 2000, 289, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Li, Y.C. Molecular mechanism of 1,25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E916–E924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Y.; Zhu, L.; He, L.; Duan, Y.; Liang, W.; Nie, Z.; Jin, Y.; Wu, X.; Fang, Y. A meta-analysis of the relationship between vitamin D deficiency and obesity. Int. J. Clin. Exp. Med. 2015, 8, 14977–14984. [Google Scholar] [PubMed]
- Saarnio, E.; Pekkinen, M.; Itkonen, S.T.; Kemi, V.; Karp, H.; Ivaska, K.K.; Risteli, J.; Koivula, M.K.; Karkkainen, M.; Makitie, O.; et al. Low free 25-hydroxyvitamin D and high vitamin D binding protein and parathyroid hormone in obese Caucasians. A complex association with bone? PLoS ONE 2018, 13, e0192596. [Google Scholar] [CrossRef] [PubMed]
- Nobre, J.L.; Lisboa, P.C.; Lima Nda, S.; Franco, J.G.; Nogueira Neto, J.F.; de Moura, E.G.; de Oliveira, E. Calcium supplementation prevents obesity, hyperleptinaemia and hyperglycaemia in adult rats programmed by early weaning. Br. J. Nutr. 2012, 107, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Nobre, J.L.; Lisboa, P.C.; Peixoto-Silva, N.; Quitete, F.T.; Carvalho, J.C.; de Moura, E.G.; de Oliveira, E. Role of vitamin D in adipose tissue in obese rats programmed by early weaning and post diet calcium. Mol. Nutr. Food Res. 2016, 60, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Xu, B.; Liu, J.; Ren, Y.; Dai, Z.; Cui, D.; Su, X.; Si, S.; Song, S.J. Vitamin D improves immune function in immunosuppressant mice induced by glucocorticoid. Biomed. Rep. 2017, 6, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.L.; Wang, N.J.; Han, B.; Li, Q.; Chen, Y.; Zhu, C.F.; Chen, Y.C.; Xia, F.Z.; Cang, Z.; Zhu, C.X.; et al. Low vitamin D levels and non-alcoholic fatty liver disease, evidence for their independent association in men in East China: A cross-sectional study (Survey on Prevalence in East China for Metabolic Diseases and Risk Factors (SPECT-China)). Br. J. Nutr. 2016, 115, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Guo, E.; Yang, J.; Li, A.; Yang, Y.; Liu, S.; Liu, A.; Jiang, X. 1,25(OH)2 D3 attenuates hepatic steatosis by inducing autophagy in mice. Obesity 2017, 25, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.G.; Liu, Y.X.; Wang, H.; Wang, B.P.; Qu, H.Q.; Wang, B.L.; Zhu, M. Active form of vitamin D ameliorates non-alcoholic fatty liver disease by alleviating oxidative stress in a high-fat diet rat model. Endocr. J. 2017, 64, 663–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, P.J.; Jirousek, M. Sirtuins: Novel targets for metabolic disease. Curr. Opin. Investig. Drugs 2008, 9, 371–378. [Google Scholar] [PubMed]
- Banks, A.S.; Kon, N.; Knight, C.; Matsumoto, M.; Gutierrez-Juarez, R.; Rossetti, L.; Gu, W.; Accili, D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008, 8, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Rajendrasozhan, S.; Yang, S.R.; Kinnula, V.L.; Rahman, I. SIRT1, an antiinflammatory and antiaging protein, is decreased in lungs of patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008, 177, 861–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinkhan, E.K.; Lang, B.Y.; Yu, B.; Wang, Y.; Jiang, C.; Fitzhugh, M.; Dahl, M.; Campbell, M.S.; Fung, C.; Malleske, D.; et al. Maternal tobacco smoke increased visceral adiposity and serum corticosterone levels in adult male rat offspring. Pediatr. Res. 2014, 76, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Kim, J.W.; Yun, H.; Choi, S.J.; Lee, S.H.; Choi, K.C.; Lim, C.W.; Lee, K.; Kim, B. Mainstream cigarette smoke accelerates the progression of nonalcoholic steatohepatitis by modulating Kupffer cell-mediated hepatocellular apoptosis in adolescent mice. Toxicol. Lett. 2016, 256, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Vitellius, G.; Trabado, S.; Bouligand, J.; Delemer, B.; Lombes, M. Pathophysiology of Glucocorticoid Signaling. Ann. Endocrinol. 2018, 79, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Catania, A.; Gatti, S.; Colombo, G.; Lipton, J.M. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol. Rev. 2004, 56, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.W.; Deng, J.; Yang, X.C.; Yang, S.Y.; Liu, B.H.; Hao, F. An implication for post-transcriptional control: Reciprocal changes of melanocortin receptor type 2 mRNA and protein expression in alopecia areata. Med. Hypotheses 2011, 76, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Massaro, J.M.; Fox, C.S.; Larson, M.G.; Keyes, M.J.; McCabe, E.L.; Robins, S.J.; O’Donnell, C.J.; Hoffmann, U.; Jacques, P.F.; et al. Adiposity, cardiometabolic risk, and vitamin D status: The Framingham Heart Study. Diabetes 2010, 59, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Drincic, A.T.; Armas, L.A.; Van Diest, E.E.; Heaney, R.P. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity 2012, 20, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Conceicao, E.P.; Moura, E.G.; Manhaes, A.C.; Carvalho, J.C.; Nobre, J.L.; Oliveira, E.; Lisboa, P.C. Calcium reduces vitamin D and glucocorticoid receptors in the visceral fat of obese male rats. J. Endocrinol. 2016, 230, 263–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strable, M.S.; Ntambi, J.M. Genetic control of de novo lipogenesis: Role in diet-induced obesity. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Proenca, A.R.; Sertie, R.A.; Oliveira, A.C.; Campana, A.B.; Caminhotto, R.O.; Chimin, P.; Lima, F.B. New concepts in white adipose tissue physiology. Braz. J. Med. Biol. Res. 2014, 47, 192–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Ping, J.; Xiang, J.; Rao, Y.S.; Zhang, W.X.; Chen, T.; Zhang, L.; Yan, Y.E. Effects of prenatal and lactation nicotine exposure on glucose homeostasis, lipogenesis and lipid metabolic profiles in mothers and offspring. Toxicol. Res. 2016, 5, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Fu, M.; Pestell, R.; Sauve, A.A. SIRT1 and endocrine signaling. Trends Endocrinol. Metab. 2006, 17, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Haigis, M.C.; Sinclair, D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. 2010, 5, 253–295. [Google Scholar] [CrossRef] [PubMed]
- Purushotham, A.; Schug, T.T.; Xu, Q.; Surapureddi, S.; Guo, X.; Li, X. Hepatocyte-specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab. 2009, 9, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.R.; Moura, E.G.; Manhaes, A.C.; Fraga, M.C.; Claudio-Neto, S.; Abreu-Villaca, Y.; Oliveira, E.; Lisboa, P.C. Concurrent maternal and pup postnatal tobacco smoke exposure in Wistar rats changes food preference and dopaminergic reward system parameters in the adult male offspring. Neuroscience 2015, 301, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Lichtensteiger, W.; Ribary, U.; Schlumpf, M.; Odermatt, B.; Widmer, H.R. Prenatal adverse effects of nicotine on the developing brain. Prog. Brain Res. 1988, 73, 137–157. [Google Scholar] [PubMed]
- Peixoto, T.C.; Moura, E.G.; de Oliveira, E.; Soares, P.N.; Guarda, D.S.; Bernardino, D.N.; Ai, X.X.; Rodrigues, V.; de Souza, G.R.; da Silva, A.J.R.; et al. Cranberry (Vaccinium macrocarpon) extract treatment improves triglyceridemia, liver cholesterol, liver steatosis, oxidative damage and corticosteronemia in rats rendered obese by high fat diet. Eur. J. Nutr. 2018, 57, 1829–1844. [Google Scholar] [CrossRef] [PubMed]
- Peixoto-Silva, N.; Conceicao, E.P.; Carvalho, J.C.; Lima, N.S.; Nogueira-Neto, J.F.; de Oliveira, E.; Moura, E.G.; Lisboa, P.C. Does bromocriptine play a role in decreasing oxidative stress for early weaned programmed obesity? Life Sci. 2014, 95, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]







| Degree of Steatosis | MALES | FEMALES | ||
|---|---|---|---|---|
| C | S | C | S | |
| Degree 0 | 100% | 100% | 100% | 100% |
| Degree 1 | 0% | 0% | 0% | 0% |
| Degree 2 | 0% | 0% | 0% | 0% |
| Degree 3 | 0% | 0% | 0% | 0% |
| Liver Lipids | MALES | |
|---|---|---|
| C | S | |
| Cholesterol Content (mg/dL) | 1.95 ± 0.07 | 1.89 ± 0.04 |
| Triglyceride Content (mg/dL) | 2.21 ± 1.12 | 2.41 ± 0.05 |
| FEMALES | ||
| C | S | |
| Cholesterol Content (mg/dL) | 1.85 ± 0.04 | 1.90 ± 0.05 |
| Triglyceride Content (mg/dL) | 1.59 ± 0.04 | 1.65 ± 0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novaes Soares, P.; Silva Tavares Rodrigues, V.; Cherem Peixoto, T.; Calvino, C.; Aparecida Miranda, R.; Pereira Lopes, B.; Peixoto-Silva, N.; Lopes Costa, L.; Claudio-Neto, S.; Christian Manhães, A.; et al. Cigarette Smoke During Breastfeeding in Rats Changes Glucocorticoid and Vitamin D Status in Obese Adult Offspring. Int. J. Mol. Sci. 2018, 19, 3084. https://doi.org/10.3390/ijms19103084
Novaes Soares P, Silva Tavares Rodrigues V, Cherem Peixoto T, Calvino C, Aparecida Miranda R, Pereira Lopes B, Peixoto-Silva N, Lopes Costa L, Claudio-Neto S, Christian Manhães A, et al. Cigarette Smoke During Breastfeeding in Rats Changes Glucocorticoid and Vitamin D Status in Obese Adult Offspring. International Journal of Molecular Sciences. 2018; 19(10):3084. https://doi.org/10.3390/ijms19103084
Chicago/Turabian StyleNovaes Soares, Patricia, Vanessa Silva Tavares Rodrigues, Thamara Cherem Peixoto, Camila Calvino, Rosiane Aparecida Miranda, Bruna Pereira Lopes, Nayara Peixoto-Silva, Luciana Lopes Costa, Sylvio Claudio-Neto, Alex Christian Manhães, and et al. 2018. "Cigarette Smoke During Breastfeeding in Rats Changes Glucocorticoid and Vitamin D Status in Obese Adult Offspring" International Journal of Molecular Sciences 19, no. 10: 3084. https://doi.org/10.3390/ijms19103084
APA StyleNovaes Soares, P., Silva Tavares Rodrigues, V., Cherem Peixoto, T., Calvino, C., Aparecida Miranda, R., Pereira Lopes, B., Peixoto-Silva, N., Lopes Costa, L., Claudio-Neto, S., Christian Manhães, A., Oliveira, E., Gaspar de Moura, E., & Cristina Lisboa, P. (2018). Cigarette Smoke During Breastfeeding in Rats Changes Glucocorticoid and Vitamin D Status in Obese Adult Offspring. International Journal of Molecular Sciences, 19(10), 3084. https://doi.org/10.3390/ijms19103084