Systemic Platelet-Activating Factor-Receptor Agonism Enhances Non-Melanoma Skin Cancer Growth
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Mice
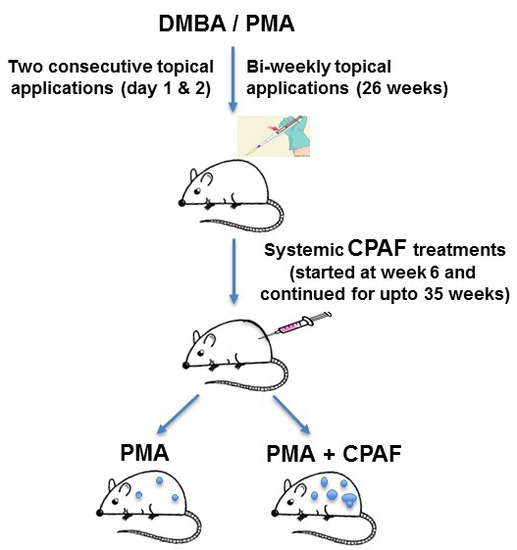
3.3. Experimental Chemical Carcinogenesis Model and Systemic CPAF Application
3.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PAF | Platelet-activating factor |
| PAF-R | Platelet-activating factor-receptor |
| CPAF | 1-hexadecyl-2-N-methyl carbamoyl glycerophosphocholine |
| DMBA | Dimethylbenz[a]anthracene |
| PMA | Phorbol 12-myristate 13-acetate |
| NMSC | Non-melanoma skin cancer |
References
- Stafforini, D.M.; McIntyre, T.M.; Zimmerman, G.A.; Prescott, S.M. Platelet activating factor, a pleiotrophic mediator of physiological and pathological processes. Crit. Rev. Clin. Lab Sci. 2003, 30, 643–672. [Google Scholar] [CrossRef] [PubMed]
- Konger, R.L.; Marathe, G.K.; Yao, Y.; Zhang, Q.; Travers, J.B. Oxidized glycerophosphocholines as biologically active mediators for ultraviolet radiation mediated effects. Prostaglandins Other Lipid Mediat. 2008, 87, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stafforini, D.M. Plasma PAF-AH (PLA2G7): Biochemical Properties, Association with LDLs and HDLs, and Regulation of Expression. Enzymes 2015, 38, 71–93. [Google Scholar] [PubMed]
- Marathe, G.K.; Pandit, C.; Lakshmikanth, C.L.; Chaithra, V.H.; Jacob, S.P.; D’Souza, C.J. To hydrolyze or not to hydrolyze: The dilemma of platelet-activating factor acetylhydrolase. J. Lipid Res. 2014, 55, 1847–1854. [Google Scholar] [CrossRef] [PubMed]
- Walterscheid, J.P.; Ullrich, S.E.; Nghiem, D.X. Platelet-activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J. Exp. Med. 2002, 195, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yao, Y.; Konger, R.L.; Sinn, A.L.; Cai, S.; Pollok, K.E.; Travers, J.B. UVB radiation-mediated inhibition of contact hypersensitivity reactions is dependent on the platelet-activating factor system. J. Invest. Dermatol. 2008, 128, 1780–1787. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.P.; Turner, M.J.; DaSilva, S.C.; Rashid, B.M.; Ocana, J.A.; Perkins, S.M.; Konger, R.L.; Touloukian, C.E.; Kaplan, M.H.; Travers, J.B. The environmental stressor ultraviolet B radiation inhibits murine antitumor immunity through its ability to generate platelet-activating factor agonists. Carcinogenesis 2012, 33, 1360–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, J.; Young, D.M.; Marentette, J.O.; Rastogi, P.; Turk, J.; McHowat, J. Lung endothelial cell platelet-activating factor production and inflammatory cell adherence are increased in response to cigarette smoke component exposure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L47–L55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehr, H.A.; Weyrich, A.S.; Saetzler, R.K.; Jurek, A.; Arfors, K.E.; Zimmerman, G.A.; Prescott, S.M.; McIntyre, T.M. Vitamin C blocks inflammatory platelet-activating factor mimetics created by cigarette smoking. J. Clin. Investig. 1997, 99, 2358–2364. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.P.; Petrache, I.; Van Demark, M.J.; Rashid, B.M.; Ocana, J.A.; Tang, Y.; Yi, Q.; Turner, M.J.; Konger, R.L.; Travers, J.B. Cigarette Smoke Exposure Inhibits Contact Hypersensitivity via the Generation of Platelet-Activating Factor Agonists. J. Immunol. 2013, 190, 2447–2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, G.; Kazimi, N.; Nghiem, D.X.; Walterscheid, J.P.; Ullrich, S.E. Platelet activating factor receptor binding plays a critical role in jet fuel-induced immune suppression. Toxicol. Appl. Pharmacol. 2004, 195, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.P.; Kozman, A.A.; Yao, Y.; DaSilva, S.C.; Rezania, S.; Martel, K.C.; Warren, S.J.; Travers, J.B.; Konger, R.L. Loss of the platelet activating factor receptor in mice augments PMA-induced inflammation and cutaneous chemical carcinogenesis. Carcinogenesis 2012, 33, 694–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa-Costa, M.; Andrade-Oliveira, V.; Braga, T.T.; Castoldi, A.; Aguiar, C.F.; Origassa, C.S.; Rodas, A.C.; Hiyane, M.I.; Malheiros, D.M.; Rios, F.J.; et al. Activation of platelet-activating factor receptor exacerbates renal inflammation and promotes fibrosis. Lab Investig. 2014, 94, 455–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ocana, J.A.; Romer, E.; Sahu, R.P.; Pawelzik, S.C.; Fitzgerald, G.; Kaplan, M.H.; Travers, J.B. Platelet-activating factor-induced reduction in contact hypersensitivity is mediated by mast cells via cyclooxygenase-2-dependent mechanisms. J. Immunol. 2018, 200, 4004–4011. [Google Scholar] [CrossRef] [PubMed]
- da Silva Junior, I.A.; Stone, S.C.; Rossetti, R.M.; Jancar, S.; Lepique, A.P. Modulation of Tumor-Associated Macrophages (TAM) Phenotype by Platelet-Activating Factor (PAF) Receptor. J. Immunol. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, E.K.; Filgueiras, L.R.; Rios, F.J.; Serezani, C.H.; Jancar, S. PAFR activation of NF-κB p65 or p105 precursor dictates pro- and anti-inflammatory responses during TLR activation in murine macrophages. Sci. Rep. 2016, 6, 32092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filgueiras, L.R.; Koga, M.M.; Quaresma, P.G.; Ishizuka, E.K.; Montes, M.B.; Prada, P.O.; Saad, M.J.; Jancar, S.; Rios, F.J. PAFR in adipose tissue macrophages is associated with anti-inflammatory phenotype and metabolic homoeostasis. Clin. Sci. (Lond.) 2016, 130, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Salinas, R.; Chen, L.; Chávez-Blanco, A.D.; Limón-Flores, A.Y.; Ma, Y.; Ullrich, S.E. An essential role for platelet-activating factor in activating mast cell migration following ultraviolet irradiation. J. Leukoc. Biol. 2014, 95, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.P.; Rezania, S.; Ocana, J.A.; DaSilva-Arnold, S.C.; Bradish, J.R.; Richey, J.D.; Warren, S.J.; Rashid, B.; Travers, J.B.; Konger, R.L. Topical application of a platelet activating factor receptor agonist suppresses phorbol ester-induced acute and chronic inflammation and has cancer chemopreventive activity in mouse skin. PLoS ONE 2014, 9, e111608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelesidis, T.; Papakonstantinou, V.; Detopoulou, P.; Fragopoulou, E.; Chini, M.; Lazanas, M.C.; Antonopoulou, S. The Role of Platelet-Activating Factor in Chronic Inflammation, Immune Activation, and Comorbidities Associated with HIV Infection. AIDS Rev. 2015, 17, 191–201. [Google Scholar] [PubMed]
- Gill, P.; Jindal, N.L.; Jagdis, A.; Vadas, P. Platelets in the immune response: Revisiting platelet-activating factor in anaphylaxis. J. Allergy. Clin. Immunol. 2015, 135, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.A.; Barcelos, L.S.; Campos, P.P.; Vasconcelos, A.C.; Teixeira, M.M.; Andrade, S.P. Sponge-induced angiogenesis and inflammation in PAF receptor-deficient mice (PAFR-KO). Br. J. Pharmacol. 2004, 141, 1185–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira, S.I.; Andrade, L.N.; Onuchic, A.C.; Nonogaki, S.; Fernandes, P.D.; Pinheiro, M.C.; Rohde, C.B.; Chammas, R.; Jancar, S. Platelet-activating factor receptor (PAF-R)-dependent pathways control tumour growth and tumour response to chemotherapy. BMC Cancer 2010, 10, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, I.A., Jr.; Chammas, R.; Lepique, A.P.; Jancar, S. Platelet-activating factor (PAF) receptor as a promising target for cancer cell repopulation after radiotherapy. Oncogenesis 2017, 6, e296. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.P.; Harrison, K.A.; Weyerbacher, J.; Murphy, R.C.; Konger, R.L.; Garrett, J.E.; Chin-Sinex, H.J.; Johnston, M.E., II; Dynlacht, J.R.; Mendonca, M.; et al. Radiation therapy generates platelet-activating factor agonists. Oncotarget 2016, 7, 20788–20800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahu, R.P.; Ocana, J.A.; Harrison, K.A.; Ferracini, M.; Touloukian, C.E.; Al-Hassani, M.; Sun, L.; Loesch, M.; Murphy, R.C.; Althouse, S.K.; et al. Chemotherapeutic agents subvert tumor immunity by generating agonists of platelet-activating factor. Cancer Res. 2014, 74, 7069–7078. [Google Scholar] [CrossRef] [PubMed]
- Filler, R.B.; Roberts, S.J.; Girardi, M. Cutaneous two-stage chemical carcinogenesis. CSH Protoc. 2007. [Google Scholar] [CrossRef] [PubMed]
- Nasti, T.H.; Cochran, J.B.; Tsuruta, Y.; Yusuf, N.; McKay, K.M.; Athar, M.; Timares, L.; Elmets, C.A. A murine model for the development of melanocytic nevi and their progression to melanoma. Mol. Carcinog. 2016, 55, 646–658. [Google Scholar] [CrossRef] [PubMed]
- Niwa, Y.; Terashima, T.; Sumi, H. Topical application of the immunosuppressant tacrolimus accelerates carcinogenesis in mouse skin. Br. J. Dermatol. 2003, 149, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.P. Expression of the platelet-activating factor receptor enhances benzyl isothiocyanate-induced apoptosis in murine and human melanoma cells. Mol. Med. Rep. 2015, 12, 394–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishii, S.; Nagase, T.; Tashiro, F.; Ikuta, K.; Sato, S.; Waga, I.; Kume, K.; Miyazaki, J.; Shimizu, T. Bronchial hyperreactivity, increased endotoxin lethality and melanocytic tumorigenesis in transgenic mice overexpressing platelet-activating factor receptor. EMBO J. 1997, 16, 133–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romer, E.; Thyagarajan, A.; Krishnamurthy, S.; Rapp, C.M.; Liu, L.; Fahy, K.; Awoyemi, A.; Sahu, R.P. Systemic Platelet-Activating Factor-Receptor Agonism Enhances Non-Melanoma Skin Cancer Growth. Int. J. Mol. Sci. 2018, 19, 3109. https://doi.org/10.3390/ijms19103109
Romer E, Thyagarajan A, Krishnamurthy S, Rapp CM, Liu L, Fahy K, Awoyemi A, Sahu RP. Systemic Platelet-Activating Factor-Receptor Agonism Enhances Non-Melanoma Skin Cancer Growth. International Journal of Molecular Sciences. 2018; 19(10):3109. https://doi.org/10.3390/ijms19103109
Chicago/Turabian StyleRomer, Eric, Anita Thyagarajan, Smita Krishnamurthy, Christine M. Rapp, Langni Liu, Katherine Fahy, Azeezat Awoyemi, and Ravi P. Sahu. 2018. "Systemic Platelet-Activating Factor-Receptor Agonism Enhances Non-Melanoma Skin Cancer Growth" International Journal of Molecular Sciences 19, no. 10: 3109. https://doi.org/10.3390/ijms19103109
APA StyleRomer, E., Thyagarajan, A., Krishnamurthy, S., Rapp, C. M., Liu, L., Fahy, K., Awoyemi, A., & Sahu, R. P. (2018). Systemic Platelet-Activating Factor-Receptor Agonism Enhances Non-Melanoma Skin Cancer Growth. International Journal of Molecular Sciences, 19(10), 3109. https://doi.org/10.3390/ijms19103109