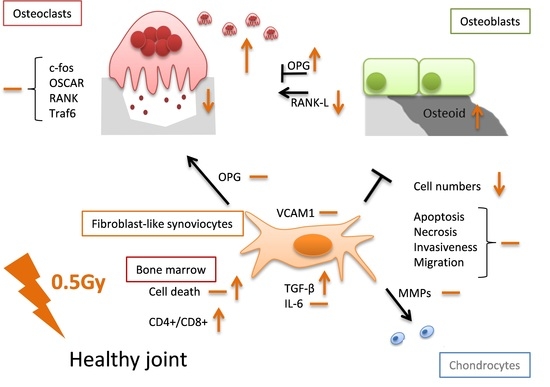
Low-Dose Radiotherapy Has No Harmful Effects on Key Cells of Healthy Non-Inflamed Joints
Abstract
:1. Introduction
2. Results
2.1. LD-RT Has a Slight Impact on FLS Cell Growth and Apoptosis but Not Invasiveness
2.2. LD-RT of 0.5 Gy Induces TGF-β Release and Has a Potential Impact on Molecules Involved in Cartillage and Bone Destruction
2.3. LD-RT Has Divergent Effects on Osteoclast Formation and Function
2.4. LD-RT Fosters Bone Formation and Temporarily Upregulates OPG Secretion in Osteoblasts
2.5. LD-RT Slighlty Increases Cell Death and Alters Immune Cell Populations in Bone-Marrow of C57bl/6 Mice
3. Discussion
4. Materials and Methods
4.1. Mouse Keeping
4.2. Ex Vivo Irradiation Procedure
4.3. Fibroblast-Like Synoviocytes (FLS) Cultures
4.4. Scratch Assay
4.5. Matrigel Invasion Assay
4.6. Osteoclast Culture
4.7. Pit Formation Assay
4.8. Osteoblast Culture
4.9. Bone Marrow Culture
4.10. Enzyme Linked Immunosorbent Assay (ELISA)
4.11. RNA and Quantitative PCR
4.12. Flow Cytometry Analyses
4.13. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACTB | β-actin |
| Acp5 | acid phosphatase 5, tartrate resistant |
| AxV | Annexinv-FITC |
| CD | Cluster of differentiation |
| cdh11 | Cadherin 11 |
| d | day |
| ELISA | Enzyme linked immunosorbent assay |
| FLS | Fibroblast-like synoviocytes |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| Gy | Gray |
| ICAM1 | Intercellular adhesion molecule 1 |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| LD-RT | Low dose-radiotherapy |
| M-CSF | Macrophage-colony stimulating factor |
| MMP | Matrixmetalloproteinase |
| NO | Nitric oxide |
| OA | Osteoarthritis |
| OC | Osteoclast |
| OPG | Osteoprotegerin |
| OSCAR | osteoclast-associated receptor |
| PBMC | Peripheral blood mononuclear cells |
| PI | Propidiumiodide |
| RA | Rheumatoid arthritis |
| RANK-L | Receptor activator of nuklear factor κ B ligand |
| RPS18 | Ribosomal protein 18 |
| RT | Radiation therapy |
| TNF-α | Tumornecrosis factor-α |
| Traf-6 | Tumor necrosis factor associated receptor-6 |
| TRAP | tartrate-resistant acid phosphatase |
| VCAM1 | Vascular adhesion molecule 1 |
References
- Derer, A.; Deloch, L.; Rubner, Y.; Fietkau, R.; Frey, B.; Gaipl, U.S. Radio-immunotherapy-induced immunogenic cancer cells as basis for induction of systemic anti-tumor immune responses—Pre-clinical evidence and ongoing clinical applications. Front. Immunol. 2015, 6, 505. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.; Hehlgans, S.; Rodel, F.; Gaipl, U.S. Modulation of inflammation by low and high doses of ionizing radiation: Implications for benign and malign diseases. Cancer Lett. 2015, 368, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Monnard. Über heilung des gelenkrheumatismus durch röntgenstrahlung bei kindern. Fortschritte auf dem Gebiet der Röntgenstrahlung 1898, 1, 209. [Google Scholar]
- Schoen, R.; Böni, A.; Miehlke, K. Klinik der Rheumatischen Erkrankungen; Springer: Berlin/Heidelberg, Germany, 1970. [Google Scholar]
- Mücke, R.; Schäfer, U. Leitlinien in der Strahlentherapie—Strahlentherapie Gutartiger Erkrankungen; Leitlinien und Empfehlungen der DEGRO: Berlin, Germany, 2013. [Google Scholar]
- Sautter-Bihl, M.L.; Liebermeister, E.; Scheurig, H.; Heinze, H.G. Analgetic irradiation of degenerative-inflammatory skeletal diseases. Benefits and risks. Dtsch. Med. Wochenschr. 1993, 118, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Ott, O.J.; Hertel, S.; Gaipl, U.S.; Frey, B.; Schmidt, M.; Fietkau, R. Benign painful shoulder syndrome: Initial results of a single-center prospective randomized radiotherapy dose-optimization trial. Strahlenther. Onkol. 2012, 188, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Ott, O.J.; Niewald, M.; Weitmann, H.D.; Jacob, I.; Adamietz, I.A.; Schaefer, U.; Keilholz, L.; Heyd, R.; Muecke, R. Degro guidelines for the radiotherapy of non-malignant disorders. Part II: Painful degenerative skeletal disorders. Strahlenther. Onkol. 2015, 191, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ott, O.J.; Hertel, S.; Gaipl, U.S.; Frey, B.; Schmidt, M.; Fietkau, R. The erlangen dose optimization trial for low-dose radiotherapy of benign painful elbow syndrome. Long-term results. Strahlenther. Onkol. 2014, 190, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Ott, O.J.; Jeremias, C.; Gaipl, U.S.; Frey, B.; Schmidt, M.; Fietkau, R. Radiotherapy for benign calcaneodynia: Long-term results of the erlangen dose optimization (EDO) trial. Strahlenther. Onkol. 2014, 190, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Ott, O.J.; Jeremias, C.; Gaipl, U.S.; Frey, B.; Schmidt, M.; Fietkau, R. Radiotherapy for benign achillodynia. Long-term results of the erlangen dose optimization trial. Strahlenther. Onkol. 2015, 191, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Rodel, F.; Frey, B.; Manda, K.; Hildebrandt, G.; Hehlgans, S.; Keilholz, L.; Seegenschmiedt, M.H.; Gaipl, U.S.; Rodel, C. Immunomodulatory properties and molecular effects in inflammatory diseases of low-dose x-irradiation. Front. Oncol. 2012, 2, 120. [Google Scholar] [CrossRef] [PubMed]
- Micke, O.; Seegenschmiedt, M.H. Consensus guidelines for radiation therapy of benign diseases: A multicenter approach in germany. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 496–513. [Google Scholar] [CrossRef]
- Seegenschmiedt, H.; Makoski, H. Strahlentherapie von schmerzen bei entzündlich degenerativen erkrankungen. Rheinisches Ärzteblatt 1999, 10/1999, 19–21. [Google Scholar]
- Arenas, M.; Sabater, S.; Hernandez, V.; Rovirosa, A.; Lara, P.C.; Biete, A.; Panes, J. Anti-inflammatory effects of low-dose radiotherapy. Indications, dose, and radiobiological mechanisms involved. Strahlenther. Onkol. 2012, 188, 975–981. [Google Scholar] [CrossRef] [PubMed]
- McKeown, S.R.; Hatfield, P.; Prestwich, R.J.; Shaffer, R.E.; Taylor, R.E. Radiotherapy for benign disease; assessing the risk of radiation-induced cancer following exposure to intermediate dose radiation. Br. J. Radiol. 2015, 88, 20150405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodel, F.; Kamprad, F.; Sauer, R.; Hildebrandt, G. Functional and molecular aspects of anti-inflammatory effects of low-dose radiotherapy. Strahlenther. Onkol. 2002, 178, 1–9. [Google Scholar] [PubMed]
- Kern, P.M.; Keilholz, L.; Forster, C.; Hallmann, R.; Herrmann, M.; Seegenschmiedt, M.H. Low-dose radiotherapy selectively reduces adhesion of peripheral blood mononuclear cells to endothelium in vitro. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2000, 54, 273–282. [Google Scholar] [CrossRef]
- Rodel, F.; Keilholz, L.; Herrmann, M.; Sauer, R.; Hildebrandt, G. Radiobiological mechanisms in inflammatory diseases of low-dose radiation therapy. Int. J. Radiat. Biol. 2007, 83, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, R.; Ernst, A.; Rodel, F.; Fietkau, R.; Ott, O.; Lauber, K.; Frey, B.; Gaipl, U.S. Low and moderate doses of ionizing radiation up to 2 Gy modulate transmigration and chemotaxis of activated macrophages, provoke an anti-inflammatory cytokine milieu, but do not impact upon viability and phagocytic function. Clin. Exp. Immunol. 2015, 179, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Arenas, M.; Gil, F.; Gironella, M.; Hernandez, V.; Jorcano, S.; Biete, A.; Pique, J.M.; Panes, J. Anti-inflammatory effects of low-dose radiotherapy in an experimental model of systemic inflammation in mice. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D.; Jahns, J.; Hildebrandt, G.; Trott, K.R. Radiation treatment of acute inflammation in mice. Int. J. Radiat. Biol. 2005, 81, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.; Ruckert, M.; Deloch, L.; Ruhle, P.F.; Derer, A.; Fietkau, R.; Gaipl, U.S. Immunomodulation by ionizing radiation-impact for design of radio-immunotherapies and for treatment of inflammatory diseases. Immunol. Rev. 2017, 280, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Cucu, A.; Shreder, K.; Kraft, D.; Ruhle, P.F.; Klein, G.; Thiel, G.; Frey, B.; Gaipl, U.S.; Fournier, C. Decrease of markers related to bone erosion in serum of patients with musculoskeletal disorders after serial low-dose radon spa therapy. Front. Immunol. 2017, 8, 882. [Google Scholar] [CrossRef] [PubMed]
- Ruhle, P.F.; Wunderlich, R.; Deloch, L.; Fournier, C.; Maier, A.; Klein, G.; Fietkau, R.; Gaipl, U.S.; Frey, B. Modulation of the peripheral immune system after low-dose radon spa therapy: Detailed longitudinal immune monitoring of patients within the RAD-ON01 study. Autoimmunity 2017, 50, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.D. Why does chronic inflammation persist: An unexpected role for fibroblasts. Immunol. Lett. 2011, 138, 12–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef] [PubMed]
- Niedermeier, M.; Pap, T.; Korb, A. Therapeutic opportunities in fibroblasts in inflammatory arthritis. Best Pract. Res. Clin. Rheumatol. 2010, 24, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Bottini, N.; Firestein, G.S. Duality of fibroblast-like synoviocytes in ra: Passive responders and imprinted aggressors. Nat. Rev. Rheumatol. 2013, 9, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.Y.; Beyer, C.; Giessl, A.; Kireva, T.; Scholtysek, C.; Uderhardt, S.; Munoz, L.E.; Dees, C.; Distler, A.; Wirtz, S.; et al. Autophagy regulates tnfalpha-mediated joint destruction in experimental arthritis. Ann. Rheum. Dis. 2013, 72, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Schwarz, E.M.; Boyce, B.F. Osteoclast precursors, rankl/rank, and immunology. Immunol. Rev. 2005, 208, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Redlich, K.; Hayer, S.; Ricci, R.; David, J.P.; Tohidast-Akrad, M.; Kollias, G.; Steiner, G.; Smolen, J.S.; Wagner, E.F.; Schett, G. Osteoclasts are essential for tnf-alpha-mediated joint destruction. J. Clin. Investig. 2002, 110, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Schett, G. Cells of the synovium in rheumatoid arthritis. Osteoclasts. Arthritis Res. Ther. 2007, 9, 203. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R. Inflammatory signaling induced bone loss. Bone 2015, 80, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Neve, A.; Corrado, A.; Cantatore, F.P. Osteoblast physiology in normal and pathological conditions. Cell Tissue Res. 2011, 343, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Maruotti, N.; Grano, M.; Colucci, S.; d’Onofrio, F.; Cantatore, F.P. Osteoclastogenesis and arthritis. Clin. Exp. Med. 2011, 11, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Haynes, D.R. Inflammatory cells and bone loss in rheumatoid arthritis. Arthritis Res. Ther. 2007, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Glantschnig, H.; Fisher, J.E.; Wesolowski, G.; Rodan, G.A.; Reszka, A.A. M-csf, tnfalpha and rank ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ. 2003, 10, 1165–1177. [Google Scholar] [CrossRef] [PubMed]
- Muller-Ladner, U.; Pap, T.; Gay, R.E.; Neidhart, M.; Gay, S. Mechanisms of disease: The molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat. Clin. Pract. Rheumatol. 2005, 1, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Schonfeld, C.; Pap, T.; Neumann, E.; Muller-Ladner, U. Fibroblasts as pathogenic cells in rheumatic inflammation. Z. Rheumatol. 2015, 74, 33–38. [Google Scholar] [PubMed]
- Muller-Ladner, U.; Kriegsmann, J.; Franklin, B.N.; Matsumoto, S.; Geiler, T.; Gay, R.E.; Gay, S. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into scid mice. Am. J. Pathol. 1996, 149, 1607–1615. [Google Scholar] [PubMed]
- Rodel, F.; Frey, B.; Gaipl, U.; Keilholz, L.; Fournier, C.; Manda, K.; Schollnberger, H.; Hildebrandt, G.; Rodel, C. Modulation of inflammatory immune reactions by low-dose ionizing radiation: Molecular mechanisms and clinical application. Curr. Med. Chem. 2012, 19, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Neumann, E.; Lefevre, S.; Zimmermann, B.; Gay, S.; Muller-Ladner, U. Rheumatoid arthritis progression mediated by activated synovial fibroblasts. Trends Mol. Med. 2010, 16, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Gu, Z.; Brenner, M.B. Fibroblast-like synoviocytes in inflammatory arthritis pathology: The emerging role of cadherin-11. Immunol. Rev. 2010, 233, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.M.; Kiener, H.P.; Agarwal, S.K.; Noss, E.H.; Watts, G.F.; Chisaka, O.; Takeichi, M.; Brenner, M.B. Cadherin-11 in synovial lining formation and pathology in arthritis. Science 2007, 315, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Gravallese, E.M.; Manning, C.; Tsay, A.; Naito, A.; Pan, C.; Amento, E.; Goldring, S.R. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000, 43, 250–258. [Google Scholar] [CrossRef]
- Charles, J.F.; Aliprantis, A.O. Osteoclasts: More than ‘bone eaters’. Trends Mol. Med. 2014, 20, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Sherrer, Y. Abatacept in biologic-naive patients and tnf inadequate responders: Clinical data in focus. Curr. Med. Res. Opin. 2008, 24, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Lin, T.; Tribble, M.B.; Zhu, J.; Altman, A.R.; Tseng, W.J.; Zhang, Y.; Akintoye, S.O.; Cengel, K.; Liu, X.S.; et al. Pth1-34 alleviates radiotherapy-induced local bone loss by improving osteoblast and osteocyte survival. Bone 2014, 67, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Deloch, L.; Derer, A.; Hueber, A.J.; Herrmann, M.; Schett, G.A.; Wölfelschneider, J.; Hahn, J.; Rühle, P.-F.; Stillkrieg, W.; Fuchs, J.; et al. Low-dose radiotherapy ameliorates advanced arthritis in hTNF-α tg mice by particularly positively impacting on bone metabolism. Front. Immunol. 2018, 9, 1834. [Google Scholar] [CrossRef] [PubMed]
- Boyce, B.F.; Yamashita, T.; Yao, Z.; Zhang, Q.; Li, F.; Xing, L. Roles for nf-kappab and c-fos in osteoclasts. J. Bone Miner. Metab. 2005, 23, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Takami, M.; Rho, J.; Josien, R.; Choi, Y. A novel member of the leukocyte receptor complex regulates osteoclast differentiation. J. Exp. Med. 2002, 195, 201–209. [Google Scholar] [CrossRef] [PubMed]
- David, J.P.; Schett, G. Tnf and bone. Curr. Dir. Autoimmun. 2010, 11, 135–144. [Google Scholar] [PubMed]
- Lomaga, M.A.; Yeh, W.C.; Sarosi, I.; Duncan, G.S.; Furlonger, C.; Ho, A.; Morony, S.; Capparelli, C.; Van, G.; Kaufman, S.; et al. Traf6 deficiency results in osteopetrosis and defective interleukin-1, cd40, and lps signaling. Genes Dev. 1999, 13, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, K.; Bollerslev, J.; Everts, V.; Karsdal, M.A. Osteoclast activity and subtypes as a function of physiology and pathology—Implications for future treatments of osteoporosis. Endocr. Rev. 2011, 32, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Huang, Q.; Xu, W.; She, C.; Xie, Z.G.; Mao, Y.T.; Dong, Q.R.; Ling, M. Low-dose x-ray irradiation promotes osteoblast proliferation, differentiation and fracture healing. PLoS ONE 2014, 9, e104016. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.K.; Kwon, J.E.; Lee, S.Y.; Lee, E.J.; Kim, D.S.; Moon, S.J.; Lee, J.; Kwok, S.K.; Park, S.H.; Cho, M.L. Il-17-mediated mitochondrial dysfunction impairs apoptosis in rheumatoid arthritis synovial fibroblasts through activation of autophagy. Cell Death Dis. 2017, 8, e2565. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Takahashi, N.; Jimi, E.; Udagawa, N.; Takami, M.; Kotake, S.; Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; et al. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J. Exp. Med. 2000, 191, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Feldmann, M.; Brennan, F.M.; Maini, R.N. Rheumatoid arthritis. Cell 1996, 85, 307–310. [Google Scholar] [CrossRef]
- Muller-Ladner, U.; Gay, S. Mmps and rheumatoid synovial fibroblasts: Siamese twins in joint destruction? Ann. Rheum. Dis. 2002, 61, 957–959. [Google Scholar] [CrossRef] [PubMed]
- Withrow, J.; Murphy, C.; Liu, Y.; Hunter, M.; Fulzele, S.; Hamrick, M.W. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res. Ther. 2016, 18, 286. [Google Scholar] [CrossRef] [PubMed]
- Willey, J.S.; Lloyd, S.A.; Robbins, M.E.; Bourland, J.D.; Smith-Sielicki, H.; Bowman, L.C.; Norrdin, R.W.; Bateman, T.A. Early increase in osteoclast number in mice after whole-body irradiation with 2 Gy X rays. Radiat. Res. 2008, 170, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Nandeesh, U.K.A.B.N. Physiology of bone formation, remodeling, and metabolism. In Radionuclide and Hybrid Bone Imaging; Fogelman, I.G., van der Wall, H.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; p. 1046. [Google Scholar]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. CJASN 2008, 3 (Suppl. 3), S131–S139. [Google Scholar] [CrossRef] [PubMed]
- Vaananen, H.K.; Zhao, H.; Mulari, M.; Halleen, J.M. The cell biology of osteoclast function. J. Cell Sci. 2000, 113 Pt 3, 377–381. [Google Scholar]
- Udagawa, N.; Takahashi, N.; Yasuda, H.; Mizuno, A.; Itoh, K.; Ueno, Y.; Shinki, T.; Gillespie, M.T.; Martin, T.J.; Higashio, K.; et al. Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology 2000, 141, 3478–3484. [Google Scholar] [CrossRef] [PubMed]
- Armaka, M.; Gkretsi, V.; Kontoyiannis, D.; Kollias, G. A standardized protocol for the isolation and culture of normal and arthritogenic murine synovial fibroblasts. Protoc. Exch. 2009. [Google Scholar] [CrossRef]
- Liang, C.; Park, A.; Guan, J. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329. [Google Scholar] [CrossRef] [PubMed]






| MWG Primers | ||
|---|---|---|
| Symbol | Forward [5′→3′] | Reverse [3′→5′] |
| Acp5 | CGACAAGAGGTTCCAGGAGA | TTCCAGCCAGCACATACCAG |
| ACTB | ACAGCTTCTTTGCAGCTCCTTCG | ATCGTCATCCATGGCGAACTGG |
| B2M | CTGCTACGTAACACAGTTCCACCC | CATGATGCTTGATCACATGTCTCG |
| catK | GGC CAG TGT GGT TCC TGT T | CAG TGG TCA TAT AGC CGC CTC |
| cdh11 | TTTCCACAGAGCGTGTACCA | GGGTCTTTAGCCTTCACCCTT |
| c-fos | CGACCATGATGTTCTCGGGT | TCGGCTGGGGAATGGTAGTA |
| GAPDH | AGGTCGGTGTGAACGGATTTG | GGGGTCGTTGATGGCAACA |
| HMBS | GAGTCTAGATGGCTCAGATAGCATGC | CCTACAGACCAGTTAGCGCACATC |
| HPRT | GTTGGGCTTACCTCACTGCTTTC | CCTGGTTCATCATCGCTAATCACG |
| MMP-3 | GCTGTCTTTGAAGCATTTGGGTT | ACAATTAAACCAGCTATTGCTCTTC |
| OSCAR | TGCCTGGAGATGGACAGAGA | GCCTGACAGTGTGGTGAAGA |
| Tnfrsf11a/RANK | GCC CAG TCT CAT CGT TCT GC | GCA AGC ATC ATT GAC CCA ATT C |
| Tnfsf11/RANK-L | ACC AGC ATC AAA ATC CCA AG | TTT GAA AGC CCC AAA GTA CG |
| TBP | TCTGAGAGCTCTGGAATTGTACCG | TGATGACTGCAGCAAATCGCTTG |
| Traf6 | AAA GCG AGA GAT TCT TTC CCT G | ACT GGG GAC AAT TCA CTA GAG C |
| VCAM-1 | CCCACCATTGAAGATACCGGG | GGGGGCAACGTTGACATAAAG |
| BioRad primers | ||
| Symbol | Unique Assay ID | |
| MMP-13 | qMmuCID0025884 | |
| RPS18 | qMmuCED0045430 | |
| Tnfrsf11b/OPG | qMmuCID0027158 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deloch, L.; Rückert, M.; Fietkau, R.; Frey, B.; Gaipl, U.S. Low-Dose Radiotherapy Has No Harmful Effects on Key Cells of Healthy Non-Inflamed Joints. Int. J. Mol. Sci. 2018, 19, 3197. https://doi.org/10.3390/ijms19103197
Deloch L, Rückert M, Fietkau R, Frey B, Gaipl US. Low-Dose Radiotherapy Has No Harmful Effects on Key Cells of Healthy Non-Inflamed Joints. International Journal of Molecular Sciences. 2018; 19(10):3197. https://doi.org/10.3390/ijms19103197
Chicago/Turabian StyleDeloch, Lisa, Michael Rückert, Rainer Fietkau, Benjamin Frey, and Udo S. Gaipl. 2018. "Low-Dose Radiotherapy Has No Harmful Effects on Key Cells of Healthy Non-Inflamed Joints" International Journal of Molecular Sciences 19, no. 10: 3197. https://doi.org/10.3390/ijms19103197
APA StyleDeloch, L., Rückert, M., Fietkau, R., Frey, B., & Gaipl, U. S. (2018). Low-Dose Radiotherapy Has No Harmful Effects on Key Cells of Healthy Non-Inflamed Joints. International Journal of Molecular Sciences, 19(10), 3197. https://doi.org/10.3390/ijms19103197