Optical Graphene-Based Biosensor for Nucleic Acid Detection; Influence of Graphene Functionalization and Ionic Strength
Abstract
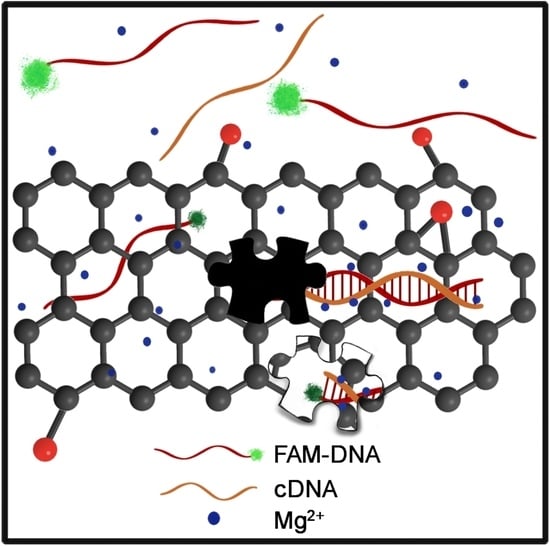
:1. Introduction
2. Results
2.1. Impact of Dispersion on Detection Platform Sensitivity
2.2. Interaction between DNA and Graphene Platforms in Aqueous Solution
2.3. Assessment of the Effects of Magnesium Ions on Fluorescence Intensity
2.4. Fluorescence Anisotropy
3. Discussion
3.1. Microstructural Characterization and Degree of Dispersion
3.2. Mechanisms of Interaction between DNA and Graphenic Platforms
3.3. A Beneficial Influence of Magnesium
3.4. Fluorescence Anisotropy
4. Materials and Methods
4.1. Preparation of Graphenic Nanomaterials Samples
4.2. Graphene Oxide (GO)-Based Biosensor Preparation
4.3. Magnesium Ions Effect
4.4. Fluorescence and Microscopy Analysis
4.5. Data Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bayat, P.; Nosrati, R.; Alibolandi, M.; Rafatpanah, H.; Abnous, K.; Khedri, M.; Ramezani, M. SELEX methods on the road to protein targeting with nucleic acid aptamers. Biochimie 2018, 154, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Geddes, C.D. Metal-enhanced fluorescence. Phys. Chem. Chem. Phys. 2013, 15, 19537. [Google Scholar] [CrossRef] [PubMed]
- Vlasceanu, G.M.; Amarandi, R.M.; Ionita, M.; Tite, T.; Iovu, H.; Pilan, L.; Burns, J.S. Versatile graphene biosensors for enhancing human cell therapy. Biosens. Bioelectron. 2018, 117, 283–302. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.M.; Kang, J.H.; Hong, B.H. Graphene-based nanomaterials for versatile imaging studies. Chem. Soc. Rev. 2015, 44, 4835–4852. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.W.; Salgado, S.; Maheshwari, V.; Liu, J.W. DNA adsorbed on graphene and graphene oxide: Fundamental interactions, desorption and applications. Curr. Opin. Colloid Interface Sci. 2016, 26, 41–49. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of graphene: Covalent and non-covalent approaches, derivatives and applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Du, D.H.; Song, H.O.; Nie, Y.T.; Sun, X.H.; Chen, L.; Ouyang, J.Y. Photoluminescence of Graphene Oxide in Visible Range Arising from Excimer Formation. J. Phys. Chem. C 2015, 119, 20085–20090. [Google Scholar] [CrossRef]
- Hasan, M.T.; Senger, B.J.; Ryan, C.; Culp, M.; Gonzalez-Rodriguez, R.; Coffer, J.L.; Naumov, A.V. Optical Band Gap Alteration of Graphene Oxide via Ozone Treatment. Sci. Rep. 2017, 7, 6411. [Google Scholar] [CrossRef] [PubMed]
- Imani, R.; Emami, S.H.; Faghihi, S. Nano-graphene oxide carboxylation for efficient bioconjugation applications: A quantitative optimization approach. J. Nanopart. Res. 2015, 17, 88. [Google Scholar] [CrossRef]
- Su, Y.; Du, J.H.; Sun, D.M.; Liu, C.; Cheng, H.M. Reduced graphene oxide with a highly restored pi-conjugated structure for inkjet printing and its use in all-carbon transistors. Nano. Res. 2013, 6, 842–852. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Kim, S.; Min, D.H. Biosensors based on graphene oxide and its biomedical application. Adv. Drug Deliv. Rev. 2016, 105, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Suvarnaphaet, P.; Pechprasarn, S. Graphene-Based Materials for Biosensors: A Review. Sensors 2017, 17, 2161. [Google Scholar] [CrossRef] [PubMed]
- Abid; Sehrawat, P.; Islam, S.S.; Mishra, P.; Ahmad, S. Reduced graphene oxide (rGO) based wideband optical sensor and the role of Temperature, Defect States and Quantum Efficiency. Sci. Rep. 2018, 8, 3537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syama, S.; Aby, C.P.; Maekawa, T.; Sakthikumar, D.; Mohanan, P.V. Nano-bio compatibility of PEGylated reduced graphene oxide on mesenchymal stem cells. 2D Mater. 2017. [Google Scholar] [CrossRef]
- Zhou, X.M.; Liao, Y.H.; Xing, D. Sensitive monitoring of RNA transcription levels using a graphene oxide fluorescence switch. Sci. Bull. 2013, 58, 2634–2639. [Google Scholar] [CrossRef]
- Huang, P.J.; Liu, J. Molecular beacon lighting up on graphene oxide. Anal. Chem. 2012, 84, 4192–4198. [Google Scholar] [CrossRef] [PubMed]
- Szöllosi, J.; Damjanovich, S.; Mátyus, L. Application of fluorescence resonance energy transfer in the clinical laboratory: Routine and research. Cytometry 1998, 34, 159–179. [Google Scholar] [CrossRef]
- Shrestha, D.; Jenei, A.; Nagy, P.; Vereb, G.; Szollosi, J. Understanding FRET as a research tool for cellular studies. Int. J. Mol. Sci. 2015, 16, 6718–6756. [Google Scholar] [CrossRef] [PubMed]
- Paul, T.; Bera, S.C.; Agnihotri, N.; Mishra, P.P. Single-Molecule FRET Studies of the Hybridization Mechanism during Noncovalent Adsorption and Desorption of DNA on Graphene Oxide. J. Phys. Chem. A 2016, 120, 11628–11636. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Liu, F.; Seo, T.S. The photoluminescent graphene oxide serves as an acceptor rather than a donor in the fluorescence resonance energy transfer pair of Cy3.5-graphene oxide. Chem. Commun. 2011, 47, 12149–12151. [Google Scholar] [CrossRef] [PubMed]
- Liscio, A.; Kouroupis-Agalou, K.; Betriu, X.D.; Kovtun, A.; Treossi, E.; Pugno, N.M.; De Luca, G.; Giorgini, L.; Palermo, V. Evolution of the size and shape of 2D nanosheets during ultrasonic fragmentation. 2D Mater. 2017. [Google Scholar] [CrossRef]
- Ye, S.B.; Feng, J.C. The effect of sonication treatment of graphene oxide on the mechanical properties of the assembled films. RSC. Adv. 2016, 6, 39681–39687. [Google Scholar] [CrossRef]
- Halbig, C.E.; Nacken, T.J.; Walter, J.; Damm, C.; Eigler, S.; Peukert, W. Quantitative investigation of the fragmentation process and defect density evolution of oxo-functionalized graphene due to ultrasonication and milling. Carbon 2016, 96, 897–903. [Google Scholar] [CrossRef]
- Coleman, B.R.; Knight, T.; Gies, V.; Jakubek, Z.J.; Zou, S. Manipulation and Quantification of Graphene Oxide Flake Size: Photoluminescence and Cytotoxicity. ACS Appl. Mater. Interfaces 2017, 9, 28911–28921. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jiao, B.N.; Tang, H.W. Interaction of single-stranded DNA with graphene oxide: Fluorescence study and its application for S1 nuclease detection. RSC. Adv. 2014, 4, 18294–18300. [Google Scholar] [CrossRef]
- Wang, S.B.; Sun, H.Q.; Ang, H.M.; Tade, M.O. Adsorptive remediation of environmental pollutants using novel graphene-based nanomaterials. Chem. Eng. J. 2013, 226, 336–347. [Google Scholar] [CrossRef]
- Hartwig, A. Role of magnesium in genomic stability. Mutat. Res. 2001, 475, 113–121. [Google Scholar] [CrossRef]
- Wu, M.; Kempaiah, R.; Huang, P.J.; Maheshwari, V.; Liu, J. Adsorption and desorption of DNA on graphene oxide studied by fluorescently labeled oligonucleotides. Langmuir 2011, 27, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Mi, L.; Zhou, X.; Chen, J.; Hu, J.; Guo, S.; Zhang, Y. Adsorption of double-stranded DNA to graphene oxide preventing enzymatic digestion. Nanoscale 2011, 3, 3888–3892. [Google Scholar] [CrossRef] [PubMed]
- Amarandi, R.M.; Becheru, D.F.; Vlasceanu, G.M.; Ionita, M.; Burns, J.S. Advantages of Graphene Biosensors for Human Stem Cell Therapy Potency Assays. Biomed. Res. Int. 2018, 2018, 1676851. [Google Scholar] [CrossRef] [PubMed]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Engineer. 2017, 184, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Shih, C.J.; Lin, S.; Sharma, R.; Strano, M.S.; Blankschtein, D. Understanding the pH-dependent behavior of graphene oxide aqueous solutions: A comparative experimental and molecular dynamics simulation study. Langmuir 2012, 28, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Todd, A.D.; Bielawski, C.W. Harnessing the chemistry of graphene oxide. Chem. Soc. Rev. 2014, 43, 5288–5301. [Google Scholar] [CrossRef] [PubMed]
- Gowtham, S.; Scheicher, R.H.; Ahuja, R.; Pandey, R.; Karna, S.P. Physisorption of nucleobases on graphene: Density-functional calculations. Phys. Rev. B 2007, 76, 33401-1–33401-4. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Kim, G.S.; Kim, S.J. Graphene nanosheets: Ultrasound assisted synthesis and characterization. Ultrason. Sonochem. 2013, 20, 644–649. [Google Scholar] [CrossRef] [PubMed]
- Geetha Bai, R.; Muthoosamy, K.; Shipton, F.N.; Manickam, S. Acoustic cavitation induced generation of stabilizer-free, extremely stable reduced graphene oxide nanodispersion for efficient delivery of paclitaxel in cancer cells. Ultrason. Sonochem. 2017, 36, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cote, L.J.; Kim, F.; Huang, J.X. Visualizing Graphene Based Sheets by Fluorescence Quenching Microscopy. J. Am. Chem. Soc. 2010, 132, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Voicu, S.I.; Pandele, M.A.; Vasile, E.; Rughinis, R.; Crica, L.; Pilan, L.; Ionita, M. The Impact of Sonication Time through Polysulfone-Graphene Oxide Composite Films Properties. Dig. J. Nanomater. Biostruct. 2013, 8, 1389–1394. [Google Scholar]
- Ciesielski, A.; Haar, S.; Aliprandi, A.; El Garah, M.; Tregnago, G.; Cotella, G.F.; El Gemayel, M.; Richard, F.; Sun, H.; Cacialli, F.; et al. Modifying the Size of Ultrasound-Induced Liquid-Phase Exfoliated Graphene: From Nanosheets to Nanodots. ACS. Nano 2016, 10, 10768–10777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, W.K.; Yoon, Y.; Song, Y.H.; Choi, S.Y.; Kim, S.; Do, Y.; Lee, J.; Park, H.; Yoon, D.H.; Yang, W.S. High-efficiency exfoliation of large-area mono-layer graphene oxide with controlled dimension. Sci. Rep. 2017, 7, 16414. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Sun, Z.; Zhang, X.; Liu, J. Mechanisms of DNA sensing on graphene oxide. Anal. Chem. 2013, 85, 7987–7993. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.H.; Yang, H.H.; Zhu, C.L.; Chen, X.; Chen, G.N. A graphene platform for sensing biomolecules. Angew. Chem. 2009, 48, 4785–4787. [Google Scholar] [CrossRef] [PubMed]
- He, S.J.; Song, B.; Li, D.; Zhu, C.F.; Qi, W.P.; Wen, Y.Q.; Wang, L.H.; Song, S.P.; Fang, H.P.; Fan, C.H. A Graphene Nanoprobe for Rapid, Sensitive, and Multicolor Fluorescent DNA Analysis. Adv. Funct. Mater. 2010, 20, 453–459. [Google Scholar] [CrossRef]
- Park, J.S.; Goo, N.I.; Kim, D.E. Mechanism of DNA adsorption and desorption on graphene oxide. Langmuir 2014, 30, 12587–12595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, P.J.; Servos, M.R.; Liu, J. Effects of polyethylene glycol on DNA adsorption and hybridization on gold nanoparticles and graphene oxide. Langmuir 2012, 28, 14330–14337. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, C.; Gang, J. Effect of pH and Salt on Adsorption of Double-Stranded DNA on Graphene Oxide. J. Nanosci. Nanotechnol. 2015, 15, 7913–7917. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Jung, J.; Kim, S.K. Flexibility of single-stranded DNA measured by single-molecule FRET. Biophys. Chem. 2014, 195, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Bekturov, E.; Tolendina, A.; Shaikhutdinov, Y.; Dzhumadilov, T. Complexation of poly (ethylene glycol) with some salts of alkali-earth metals. Polym. Adv. Technol. 1993, 4, 564–566. [Google Scholar] [CrossRef]
- Liu, Z.B.; Liu, B.W.; Ding, J.S.; Liu, J.W. Fluorescent sensors using DNA-functionalized graphene oxide. Anal. Bioanal. Chem. 2014, 406, 6885–6902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruscito, A.; DeRosa, M.C. Small-Molecule Binding Aptamers: Selection Strategies, Characterization, and Applications. Front. Chem. 2016, 4, 14. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becheru, D.F.; Vlăsceanu, G.M.; Banciu, A.; Vasile, E.; Ioniţă, M.; Burns, J.S. Optical Graphene-Based Biosensor for Nucleic Acid Detection; Influence of Graphene Functionalization and Ionic Strength. Int. J. Mol. Sci. 2018, 19, 3230. https://doi.org/10.3390/ijms19103230
Becheru DF, Vlăsceanu GM, Banciu A, Vasile E, Ioniţă M, Burns JS. Optical Graphene-Based Biosensor for Nucleic Acid Detection; Influence of Graphene Functionalization and Ionic Strength. International Journal of Molecular Sciences. 2018; 19(10):3230. https://doi.org/10.3390/ijms19103230
Chicago/Turabian StyleBecheru, Diana F., George M. Vlăsceanu, Adela Banciu, Eugeniu Vasile, Mariana Ioniţă, and Jorge S. Burns. 2018. "Optical Graphene-Based Biosensor for Nucleic Acid Detection; Influence of Graphene Functionalization and Ionic Strength" International Journal of Molecular Sciences 19, no. 10: 3230. https://doi.org/10.3390/ijms19103230
APA StyleBecheru, D. F., Vlăsceanu, G. M., Banciu, A., Vasile, E., Ioniţă, M., & Burns, J. S. (2018). Optical Graphene-Based Biosensor for Nucleic Acid Detection; Influence of Graphene Functionalization and Ionic Strength. International Journal of Molecular Sciences, 19(10), 3230. https://doi.org/10.3390/ijms19103230