Hydrogel Cryopreservation System: An Effective Method for Cell Storage
Abstract
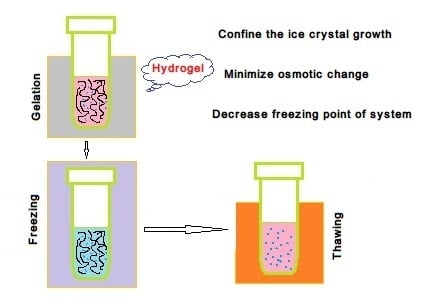
:1. Introduction
2. Natural Polymer Hydrogel Cryopreservation System
2.1. Alginate Hydrogel Cryopreservation System
2.2. Chitosan/Alginate Hydrogel Cryopreservation System
3. Synthetic Polymer Hydrogel Cryopreservation System
4. Supramolecular Hydrogel Cryopreservation System
5. Materials and Methods
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mandal, A.; Raju, S.; Viswanathan, C. Long-term culture and cryopreservation does not affect the stability and functionality of human embryonic stem cell-derived hepatocyte-like cells. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.D.; Ratcliffe, E.; Hourd, P.; Williams, D.J.; Thomas, R.J. A quality-by-design approach to risk reduction and optimization for human embryonic stem cell cryopreservation processes. Tissue Eng. Part C Methods 2014, 20, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-Y.; Yang, Y.-C.; Hung, S.-H.; Lee, S.-Y.; Lee, M.-S.; Chu, I.M.; Hwang, S.-M. Cryopreservation of human embryonic stem cells by a programmed freezer with an oscillating magnetic field. Cryobiology 2013, 66, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.; Whyte, J.; Taylor, L.; Sherman, A.; Nair, V.; Kaiser, P.; McGrew, M.J. Cryopreservation of specialized chicken lines using cultured primordial germ cells. Poult. Sci. 2016, 95, 1905–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, S.-J.; Kim, B.-G.; Lee, Y.-A.; Kim, Y.-H.; Kim, B.-J.; Jung, S.-E.; Pang, M.-G.; Ryu, B.-Y. Effect of antioxidants and apoptosis inhibitors on cryopreservation of murine germ cells enriched for spermatogonial stem cells. PLoS ONE 2016, 11, e0161372. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Yin, Y.; Zhang, L.; Hu, W.; Zhang, C.; Chen, W. A supramolecular gel approach to minimize the neural cell damage during cryopreservation process. Macromol. Biosci. 2016, 16, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Iwanami, A.; Kohyama, J.; Itakura, G.; Kawabata, S.; Sugai, K.; Nishimura, S.; Kashiwagi, R.; Yasutake, K.; Isoda, M.; et al. Safe and efficient method for cryopreservation of human induced pluripotent stem cell-derived neural stem and progenitor cells by a programmed freezer with a magnetic field. Neurosci. Res. 2016, 107, 20–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porozhan, I.A.; Goltsev, A.N.; Babenko, N.N.; Ostankov, M.V.; Int Inst, R. Cryopreservation of fetal neural cells. In Proceedings of the 13th Cryogenics 2014 IIR International Conference, Prague, Czech Republic, 7–11 April 2014; pp. 201–206. [Google Scholar]
- Yamazaki, T.; Enosawa, S.; Tokiwa, T. Effect of cryopreservation on the appearance and liver function of hepatocyte-like cells in cultures of cirrhotic liver of biliary atresia. In Vitro Cell. Dev. Biol. Anim. 2018, 54, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Jitraruch, S.; Dhawan, A.; Hughes, R.D.; Filippi, C.; Lehec, S.C.; Glover, L.; Mitry, R.R. Cryopreservation of hepatocyte microbeads for clinical transplantation. Cell Transplant. 2017, 26, 1341–1354. [Google Scholar] [CrossRef] [PubMed]
- Stokich, B.; Osgood, Q.; Grimm, D.; Moorthy, S.; Chakraborty, N.; Menze, M.A. Cryopreservation of hepatocyte (hepg2) cell monolayers: Impact of trehalose. Cryobiology 2014, 69, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.J. Cryopreservation of human stem cells for clinical application: A review. Transfus. Med. Hemother. 2011, 38, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Meryman, H.T. Cryopreservation of living cells: Principles and practice. Transfusion 2007, 47, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, R.; Li, L.; Peters, B.M.; Li, B.; Lin, C.-W.; Chuang, T.-L.; Chen, D.; Zhao, X.; Xiong, Z.; et al. Viable but non-culturable state and toxin gene expression of enterohemorrhagic escherichia coli 0157 under cryopreservation. Res. Microbiol. 2017, 168, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Rienzi, L.; Gracia, C.; Maggiulli, R.; LaBarbera, A.R.; Kaser, D.J.; Ubaldi, F.M.; Vanderpoel, S.; Racowsky, C. Oocyte, embryo and blastocyst cryopreservation in art: Systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update 2017, 23, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Edgar, D.H.; Gook, D.A. A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos. Hum. Reprod. Update 2012, 18, 536–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manuchehrabadi, N.; Gao, Z.; Zhang, J.; Ring, H.L.; Shao, Q.; Liu, F.; McDermott, M.; Fok, A.; Rabin, Y.; Brockbank, K.G.M.; et al. Improved tissue cryopreservation using inductive heating of magnetic nanoparticles. Sci. Transl. Med. 2017, 9, eaah4586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Fuller, B.J. Cryoprotectants: The essential antifreezes to protect life in the frozen state. Cryoletters 2004, 25, 375–388. [Google Scholar] [PubMed]
- Ma, X.-H.; Shi, Y.; Hou, Y.; Liu, Y.; Zhang, L.; Fan, W.-X.; Ge, D.; Liu, T.-Q.; Cui, Z.-F. Slow-freezing cryopreservation of neural stem cell spheres with different diameters. Cryobiology 2010, 60, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Tanpradit, N.; Comizzoli, P.; Srisuwatanasagul, S.; Chatdarong, K. Positive impact of sucrose supplementation during slow freezing of cat ovarian tissues on cellular viability, follicle morphology, and DNA integrity. Theriogenology 2015, 83, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Talwar, P.; Prakash, V. Clinical Applications of Vitrification. Vitrification in Assisted Reproduction; Springer: New Delhi, India, 2015; pp. 51–63. Available online: https://link.springer.com/chapter/10.1007/978-81-322-1527-1_6 (accessed on 23 October 2018).
- Guven, S.; Demirci, U. Integrating nanoscale technologies with cryogenics: A step towards improved biopreservation. Nanomedicine 2012, 7, 1787–1789. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; McGrath, J. Freezing osteoblast cells attached to hydroxyapatite discs and glass coverslips: Mechanisms of damage. Sci. China E-Technol. Sci. 2007, 50, 248–256. [Google Scholar] [CrossRef]
- Zhurova, M.; Woods, E.J.; Acker, J.P. Intracellular ice formation in confluent monolayers of human dental stem cells and membrane damage. Cryobiology 2010, 61, 133–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takamatsu, H.; Kawahara, S.; Kurokawa, T.; Honda, H. Osmotic volume change and cellular injury by hypertonic sodium choloride solution. Trans. Jpn. Soc. Mech. Eng. B 2002, 68, 2320–2326. [Google Scholar] [CrossRef]
- Chen, W.; Lisowski, M.; Khalil, G.; Sweet, I.R.; Shen, A.Q. Microencapsulated 3-dimensional sensor for the measurement of oxygen in single isolated pancreatic islets. PLoS ONE 2012, 7, e33070. [Google Scholar] [CrossRef] [PubMed]
- Pravdyuk, A.I.; Petrenko, Y.A.; Fuller, B.J.; Petrenko, A.Y. Cryopreservation of alginate encapsulated mesenchymal stromal cells. Cryobiology 2013, 66, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Banc, A.; Woodruff, T.K.; Shea, L.D. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol. Bioeng. 2009, 103, 378–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurruchaga, H.; Ciriza, J.; Saenz del Burgo, L.; Roberto Rodriguez-Madoz, J.; Santos, E.; Prosper, F.; Maria Hernandez, R.; Orive, G.; Luis Pedraz, J. Cryopreservation of microencapsulated murine mesenchymal stem cells genetically engineered to secrete erythropoietin. Int. J. Pharmaceut. 2015, 485, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Song, H.; Zheng, H.; Ren, Y.; Li, S.; Liu, X.; Yu, W.; Ma, X. Culture of low density E. coli cells in alginate-chitosan microcapsules facilitates stress resistance by up-regulating luxs/ai-2 system. Carbohydr. Polym. 2016, 141, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey, H.Y.; Faloci, M.; Medina, R.; Dolce, N.; Engelmann, F.; Mroginski, L. Cryopreservation of arachis pintoi (leguminosae) somatic embryos. Cryo Lett. 2013, 34, 571–582. [Google Scholar]
- Benson, E.E.; Harding, K.; Ryan, M.; Petrenko, A.; Petrenko, Y.; Fuller, B. Alginate encapsulation to enhance biopreservation scope and success: A multidisciplinary review of current ideas and applications in cryopreservation and non-freezing storage. Cryoletters 2018, 39, 14–38. [Google Scholar] [PubMed]
- Schneider, S.; Klein, H.H. Long-term graft function of cryostored alginate encapsulated rat islets. Eur. J. Med. Res. 2011, 16, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Choi, J.K.; Rao, W.; Zhao, S.; Agarw0al, P.; Zhao, G.; He, X. Alginate hydrogel microencapsulation inhibits devitrification and enables large-volume low-CPA cell vitrification. Adv. Funct. Mater. 2015, 25, 6839–6850. [Google Scholar] [CrossRef] [PubMed]
- Perteghella, S.; Gaviraghi, A.; Cenadelli, S.; Bornaghi, V.; Galli, A.; Crivelli, B.; Vigani, B.; Vigo, D.; Chlapanidas, T.; Faustini, M.; et al. Alginate encapsulation preserves the quality and fertilizing ability of mediterranean Italian water buffalo (bubalus bubalis) and holstein friesian (bos taurus) spermatozoa after cryopreservation. J. Vet. Sci. 2017, 18, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wright, B.; Sahoo, R.; Connon, C.J. A novel alternative to cryopreservation for the short-term storage of stem cells for use in cell therapy using alginate encapsulation. Tissue Eng. Part. C-Methods 2013, 19, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shu, Z.; Gao, D.; Shen, A.Q. Sensing and sensibility: Single-islet-based quality control assay of cryopreserved pancreatic islets with functionalized hydrogel microcapsules. Adv. Healthc. Mater. 2016, 5, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Correia, C.; Malpique, R.; Brito, C.; Jensen, J.; Bjorquist, P.; Carrondo, M.J.T.; Alves, P.M. Microencapsulation technology: A powerful tool for integrating expansion and cryopreservation of human embryonic stem cells. PLoS ONE 2011, 6, e23212. [Google Scholar] [CrossRef] [PubMed]
- Hang, H.; Shi, X.; Gu, G.X.; Wu, Y.; Gu, J.; Ding, Y. In vitro analysis of cryopreserved alginate-poly-l-lysine-alginate-microencapsulated human hepatocytes. Liver Int. 2010, 30, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Murua, A.; Orive, G.; Hernandez, R.M.; Luis Pedraz, J. Cryopreservation based on freezing protocols for the long-term storage of microencapsulated myoblasts. Biomaterials 2009, 30, 3495–3501. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.R.; Kuo, Y.C.; Ku, H.F.; Hsieh, W.H. Cryopreserved chondrocytes in porous biomaterials with surface elastin and poly-l-lysine for cartilage regeneration. Colloids Surf. B Biointerfaces 2013, 103, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Kumar, R.S.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Effect of cryopreservation and microencapsulation of lactic acid bacterium enterococcus faecium mc13 for long-term storage. Biochem. Eng. J. 2011, 58–59, 140–147. [Google Scholar] [CrossRef]
- Durkut, S.; Elcin, A.E.; Elcin, Y.M. In vitro evaluation of encapsulated primary rat hepatocytes pre- and post-cryopreservation at-80 degrees c and in liquid nitrogen. Artif. Cells Nanomed. Biotechnol. 2015, 43, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wen, F.; Gouk, S.S.; Lee, E.H.; Kuleshova, L.L. Cryopreservation strategy for tissue engineering constructs consisting of human mesenhymal stem cells and hydrogel biomaterials. Cryoletters 2015, 36, 325–335. [Google Scholar] [PubMed]
- Ahmad, H.F.; Sambanis, A. Cryopreservation effects on recombinant myoblasts encapsulated in adhesive alginate hydrogels. Acta Biomater. 2013, 9, 6814–6822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambu, S.; Xu, X.; Schiffter, H.A.; Cui, Z.F.; Ye, H. Rgds-fuctionalized alginates improve the survival rate of encapsulated embryonic stem cells during cryopreservation. Cryoletters 2011, 32, 389–401. [Google Scholar] [PubMed]
- Wang, X.; Xu, H. Incorporation of dmso and dextran-40 into a gelatin/alginate hydrogel for controlled assembled cell cryopreservation. Cryobiology 2010, 61, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Katsen-Globa, A.; Meiser, I.; Petrenko, Y.A.; Ivanov, R.V.; Lozinsky, V.I.; Zimmermann, H.; Petrenko, A.Y. Towards ready-to-use 3-d scaffolds for regenerative medicine: Adhesion-based cryopreservation of human mesenchymal stem cells attached and spread within alginate-gelatin cryogel scaffolds. J. Mater. Sci. Mater. Med. 2014, 25, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; Kim, M.; Kim, T.-H.; Kim, J.-H.; Lee, J.H.; Park, J.-H.; Knowles, J.C.; Kim, H.-W. Utilizing core-shell fibrous collagen-alginate hydrogel cell delivery system for bone tissue engineering. Tissue Eng. Part. A 2014, 20, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Kumar, R.S.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Cryopreservation and microencapsulation of a probiotic in alginate-chitosan capsules improves survival in simulated gastrointestinal conditions. Biotechnol. Bioproc. Eng. 2011, 16, 1106–1114. [Google Scholar] [CrossRef]
- Hardikar, A.A.; Risbud, M.V.; Bhonde, R.R. Improved post-cryopreservation recovery following encapsulation of islets in chitosan-alginate microcapsules. Transpl. Proc. 2000, 32, 824–825. [Google Scholar] [CrossRef]
- Haque, T.; Chen, H.; Ouyang, W.; Martoni, C.; Lawuyi, B.; Urbanska, A.M.; Prakash, S. In vitro study of alginate-chitosan microcapsules: An alternative to liver cell transplants for the treatment of liver failure. Biotechnol. Lett. 2005, 27, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C. Recent advances in crosslinking chemistry of biomimetic poly(ethylene glycol) hydrogels. Rsc. Adv. 2015, 5, 39844–39853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Mawatari, K.; Konno, T.; Kitamori, T.; Ishihara, K. Spontaneous packaging and hypothermic storage of mammalian cells with a cell-membrane-mimetic polymer hydrogel in a microchip. ACS Appl. Mater. Int. 2015, 7, 23089–23097. [Google Scholar] [CrossRef] [PubMed]
- Vrana, N.E.; O’Grady, A.; Kay, E.; Cahill, P.A.; McGuinness, G.B. Cell encapsulation within pva-based hydrogels via freeze-thawing: A one-step scaffold formation and cell storage technique. J. Tissue Eng. Regen. Med. 2009, 3, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Rajan, R.; Hyon, S.-H.; Matsumura, K. Hydrogelation of dextran-based polyampholytes with cryoprotective properties via click chemistry. Biomater. Sci. 2014, 2, 308–317. [Google Scholar] [CrossRef]
- Fisher, J.P.; Jo, S.; Mikos, A.G.; Reddi, A.H. Thermoreversible hydrogel scaffolds for articular cartilage engineering. J. Biomed. Mater. Res. A 2004, 71A, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Drasar, P.; Budesinsky, M.; Reschel, M.; Pouzar, V.; Cerny, I. Etienic etienate as synthon for the synthesis of steroid oligoester gelators. Steroids 2005, 70, 615–625. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Maruyama, Y.; Hanabusa, K. Gel-solution phase transition of organogels with photoreversibility: L-amino acid organogelators with azobenzene. Tetrahedron. Lett. 2016, 57, 3540–3543. [Google Scholar] [CrossRef]
- Mukai, M.; Minamikawa, H.; Aoyagi, M.; Asakawa, M.; Shimizu, T.; Kogiso, M. A hydro/organo/hybrid gelator: A peptide lipid with turning aspartame head groups. J. Colloid. Interf. Sci. 2013, 395, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yin, M.; Ren, X.; Feng, Q.; Wang, J.; Zhou, Y. A coordination polymeric gelator based on ag(i) and 2, 7-bis(1-imidazole)fluorene: Synthesis, characterization, gelation and antibacterial properties. Mater. Lett. 2015, 139, 141–144. [Google Scholar] [CrossRef]
- Gao, D.; Xue, M.; Peng, J.; Liu, J.; Yan, N.; He, P.; Fang, Y. Preparation and gelling properties of sugar-contained low-molecular-mass gelators: Combination of cholesterol and linear glucose. Tetrahedron 2010, 66, 2961–2968. [Google Scholar] [CrossRef]
- Hsu, S.-M.; Chang, J.-W.; Wu, F.-Y.; Lin, Y.-C.; Lai, T.-S.; Cheng, H.; Lin, H.-C. A supramolecular hydrogel self-assembled from pentafluorobenzyl-dipeptide. RSC Adv. 2015, 5, 32431–32434. [Google Scholar] [CrossRef]
- Sarkar, K.; Dastidar, P. Supramolecular hydrogel derived from a c-3-symmetric boronic acid derivative for stimuli-responsive release of insulin and doxorubicin. Langmuir 2018, 34, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jin, M.; Chen, Y.; Gao, H.; Shi, X.; Cheng, W.; Ren, L.; Wang, Y. High internal phase emulsions stabilised by supramolecular cellulose nanocrystals and their application as cell-adhesive macroporous hydrogel monoliths. J. Mater. Chem. B 2017, 5, 2671–2678. [Google Scholar] [CrossRef]
- Liu, K.L.; Zhang, Z.; Li, J. Supramolecular hydrogels based on cyclodextrin-polymer polypseudorotaxanes: Materials design and hydrogel properties. Soft Matter 2011, 7, 11290–11297. [Google Scholar] [CrossRef]
- Wang, L.; Shi, X.; Wu, Y.; Zhang, J.; Zhu, Y.; Wang, J. A multifunctional supramolecular hydrogel: Preparation, properties and molecular assembly. Soft Matter 2018, 14, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, M.; Staropoli, M.; Appavou, M.-S.; Wyss, H.M.; Pyckhout-Hintzen, W.; Sijbesma, R.P. Tough supramolecular hydrogel based on strong hydrophobic interactions in a multiblock segmented copolymer. Macromolecules 2017, 50, 3333–3346. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, L.; Fan, Y.; Li, Y.; Cheng, L.; Liu, W.; Li, X.; Fan, X. Formation and controlled drug release using a three-component supramolecular hydrogel for anti-Schistosoma Japonicum cercariae. Nanomaterials 2016, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Chen, X.; Li, P.; Zou, W.; Wu, L.; Chen, W. Using a novel supramolecular gel cryopreservation system in microchannel to minimize the cell injury. Langmuir 2018, 34, 5088–5096. [Google Scholar] [CrossRef] [PubMed]







| Citation | Hydrogel Cryopreservation System | Main Materials | Cyropreservation | Viability |
|---|---|---|---|---|
| Huang et al., 2015 [36] | Alginate | mESCs/hADSCs; PROH; trehalose | Freezing: −60 °C; Thawing: ~24 °C; Rate: both 60 °C/min | Most of the microencapsulated cells could survive |
| Xu et al., 2009 [29] | Alginate | Ovaries 1; Follicles; Sucrose; DMSO | Freezing: (1) 4 ~ −9 °C at −2 °C/min; (2) 6 min at −9 °C; (3) seeded manually for 4 min at −9 °C; (4) −9 ~ −40 °C at −0.3 °C/min; Thawing: ~22 °C for 30 s; plunged into a 37 °C water bath with gentle shaking | Fresh-In: 78.0 ±7.7% 2; Cryo-Ov: 71.7 ± 10.9%; Cryo-In: 73.7 ±13.8% |
| Perteghella et al., 2017 [37] | Alginate | May–July: Mediterranean Italian water buffalo (B. bubalis); Holstein Friesian bulls (Bos taurus); BULLXcell extender solution | Freezing: +4 °C~ −10 °C (rate: −5 °C/min in bovine, −3 °C/min in buffalo), −10 °C ~ −100 °C (rate: −30 °C/min in bovine, −40 °C/min in buffalo), and −100 °C ~ −140 °C (rate, −20 °C/min in bovine, −20 °C/min in buffalo); Thawing: 37 °C for 1 min | No relative test |
| Chen et al., 2013 [38] | Alginate | Mouse ESCs;DMEMIsopropyl alcohol | Mouse ESCs and hMSCs: stored at room temperature (18 °C ~ 22 °C, atmospheric CO2); ~ −80 °C for overnight (rate: 1 °C/min); Thawing: incubation for 4 min in the alginate-dissolving buffer | Mouse ESCs: 74%; hMSCs: 80% |
| Chen et al., 2016 [39] | Alginate | Islets; oxygen-sensitive dye Pt (II) meso-tetra (N-methyl-4-pyridyl) porphine tetrachloride; DMSO; Trehalose | Freezing: 4 °C ~ −80 °C (rate: −1 °C/min); Thawing: quickly thawed in water bath (37 °C) | No relative test |
| Serra et al., 2011 [40] | Alginate | hESCs; DMEM; Isopropanol; DMSO | Freezing: 4 °C ~ −80 °C (rate: −1 °C/min); Thawing: quickly thawed in water bath (37 °C) | Over 70% |
| Kanmani et al., 2011 [52] | Chitosan/Alginate | probiotic bacterium S. phocae PI80; glucose, sucrose, trehalose, galactose, glycerol | Freezing: room temperature ~ −20 °C; −20 °C dried under vacuum for 20 h; stored at −20, 4, 25, and 35 °C for six months; Thawing: thawed at room temperature for 1 h | S. phocae PI80: 74.6 ± 5.9% after stored at −20 °C for six months |
| Hardikar et al., 2000 [53] | Chitosan/Alginate | Islets; DMSO | Freezing: 22 °C ~ 0 °C for 25 min; 0 °C ~ −7.5 °C for 5 min; Thawing: quickly thawed in water bath (37 °C) | Encapsulated islets: 95.4 ± 1.3% |
| Xu et al., 2015 [56] | Synthetic polymer | L929 cells; DMEM; PMBV | Freezing: room temperature ~ 4 °C; Thawing: quickly thawed in room temperature | No relative test |
| Vrana et al., 2009 [57] | Synthetic polymer | bovine thoracic arterial smooth muscle cells; PVA | Freezing: 4 °C for 1 h; −70 °C overnight; Thawing: thawed in water bath (37 °C) for 10 min; prewarmed serum was added to remove DMSO every 15 min | 50% (affected by the concentration of the serum and DMSO) |
| Jain et al., 2014 [58] | Synthetic polymer | L929 cells; DMEM; dextran-based polyampholyte; DMSO | Freezing: −80 °C overnight; Thawing: quickly thawed | [4:1(mass ratio) azide-Dex-PA(0.69): DBCO-Dex]: 93 ± 4.2% |
| Zeng et al., 2016 [6] | Supramolecular | Boc-l-tyrosine methyl ester; 1-bromododecane; PC12 cells; Schwann cells; DMSO | Freezing: 4 ~ −80 °C (rate: −1 °C/min); 0 °C ~ −7.5 °C for 5 min; Thawing: quickly thawed in water bath (37 °C) | PC12 cells: 10.2%; Schwann cells: 11.1% |
| Lan et al., 2018 [72] | Supramolecular | Boc-l-tyrosine methyl ester; 1-bromododecane; RSC96 cells; DMSO; Ethylene glycol; Trehalose | Freezing: 4 °C ~ −80 °C (rate: −1 °C/min); 0 °C ~ −7.5 °C for 5 min; Thawing: quickly thawed in water bath (37 °C) | 62.5% ~ 83.9% in different cryoprotectants |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhou, Y.; Zhang, L.; Wu, L.; Chen, Y.; Xie, D.; Chen, W. Hydrogel Cryopreservation System: An Effective Method for Cell Storage. Int. J. Mol. Sci. 2018, 19, 3330. https://doi.org/10.3390/ijms19113330
Zhang C, Zhou Y, Zhang L, Wu L, Chen Y, Xie D, Chen W. Hydrogel Cryopreservation System: An Effective Method for Cell Storage. International Journal of Molecular Sciences. 2018; 19(11):3330. https://doi.org/10.3390/ijms19113330
Chicago/Turabian StyleZhang, Chaocan, Youliang Zhou, Li Zhang, Lili Wu, Yanjun Chen, Dong Xie, and Wanyu Chen. 2018. "Hydrogel Cryopreservation System: An Effective Method for Cell Storage" International Journal of Molecular Sciences 19, no. 11: 3330. https://doi.org/10.3390/ijms19113330
APA StyleZhang, C., Zhou, Y., Zhang, L., Wu, L., Chen, Y., Xie, D., & Chen, W. (2018). Hydrogel Cryopreservation System: An Effective Method for Cell Storage. International Journal of Molecular Sciences, 19(11), 3330. https://doi.org/10.3390/ijms19113330