OsYSL13 Is Involved in Iron Distribution in Rice
Abstract
:1. Introduction
2. Results
2.1. Expression and Subcellular Localization of OsYSL13
2.2. Phenotypic Analysis of the osysl13 Mutant and OsYSL13 Overexpression Lines
2.3. Physiological Functional Analysis of OsYSL13 in Fe Homeostasis
3. Discussion
3.1. OsYSL13 Is Localized to the Plasma Membrane
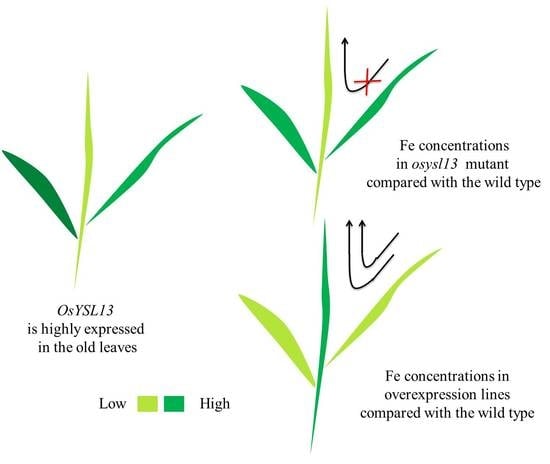
3.2. OsYSL13 Is Involved in Fe Distribution to the Youngest Leaf
3.3. OsYSL13 May Be Involved in Fe Accumulation in the Roots
3.4. OsYSL13 Is Involved in Fe Distribution to the Seeds
4. Materials and Methods
4.1. Plant Materials
4.2. Growth Conditions
4.3. Gene Expression Analysis
4.4. Subcellular Localization
4.5. Measurement of SPAD Values
4.6. Determination of Fe Concentrations
4.7. Statistical Analysis
4.8. Accession Number
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| DMA | deoxymugineic acid |
| ICP-MS | inductively coupled plasma mass spectrometry |
| IRT | iron-regulated transporter |
| MIT | mitochondrial iron transporter |
| MNM | micronutrient malnutrition |
| NA | nicotianamine |
| NRAMP | natural resistance associated macrophage protein |
| OX | overexpression |
| SPAD | soil and plant analyzer development |
| sGFP | synthetic green fluorescent protein |
| SUT | sucrose transporter |
| VIT | vacuolar iron transporter |
| YSL | yellow stripe-like |
References
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of nutrients: Micronutrients. In Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 191–248. ISBN 978-0-12-304905-2. [Google Scholar]
- Kobayashi, T.; Nishizawa, N.K. Iron uptake, translocation, and regulation in higher plants. Annu. Rev. Plant Biol. 2012, 63, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Nishizawa, N.; Hayashi, H.; Chino, M.; Yoshimura, E.; Ishihara, J. Why are young rice plants highly susceptible to iron deficiency? Plant Soil 1991, 130, 143–156. [Google Scholar] [CrossRef]
- Connolly, E.L.; Guerinot, M.L. Iron stress in plants. Genome Biol. 2002, 3, 1–4. [Google Scholar] [CrossRef]
- Curie, C.; Cassin, G.; Couch, D.; Divol, F.; Higuchi, K.; Le Jean, M.; Misson, J.; Schikora, A.; Czernic, P.; Mari, S. Metal movement within the plant: Contribution of nicotianamine and yellow stripe 1-like transporters. Ann. Bot. 2009, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.L.; Williams, L.E. Transition metal transporters in plants. J. Exp. Bot. 2003, 54, 2601–2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, C.M.; Guerinot, M.L. Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat. Chem. Biol. 2009, 5, 333–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, L.A.; Pierson, A.J.; Panaviene, Z.; Walker, E.L. Yellow stripe1. Expanded roles for the maize iron-phytosiderophore transporter. Plant Physiol. 2004, 135, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Curie, C.; Panaviene, Z.; Loulergue, C.; Dellaporta, S.L.; Briat, J.F.; Walker, E.L. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 2001, 409, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.-H.; Chiecko, J.; Punshon, T.; Lanzirotti, A.; Lahner, B.; Salt, D.E.; Walker, E.L. Successful reproduction requires the function of Arabidopsis yellow stripe-like1 and yellow stripe-like3 metal-nicotianamine transporters in both vegetative and reproductive structures. Plant Physiol. 2010, 154, 197–210. [Google Scholar] [CrossRef] [PubMed]
- DiDonato, R.J.; Roberts, L.A.; Sanderson, T.; Eisley, R.B.; Walker, E.L. Arabidopsis Yellow Stripe-Like2 (YSL2): A metal-regulated gene encoding a plasma membrane transporter of nicotianamine-metal complexes. Plant J. 2004, 39, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Kobayashi, T.; Nozoye, T.; Takahashi, M.; Kakei, Y.; Suzuki, K.; Nakazono, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. Rice OsYSL15 is an iron-regulated iron(III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J. Biol. Chem. 2009, 284, 3470–3479. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, Y.; Masuda, H.; Bashir, K.; Inoue, H.; Tsukamoto, T.; Takahashi, M.; Nakanishi, H.; Aoki, N.; Hirose, T.; Ohsugi, R.; et al. Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J. 2010, 62, 379–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koike, S.; Inoue, H.; Mizuno, D.; Takahashi, M.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J. 2004, 39, 415–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakei, Y.; Ishimaru, Y.; Kobayashi, T.; Yamakawa, T.; Nakanishi, H.; Nishizawa, N.K. OsYSL16 plays a role in the allocation of iron. Plant Mol. Biol. 2012, 79, 583–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Jean, M.; Schikora, A.; Mari, S.; Briat, J.F.; Curie, C. A loss-of-function mutation in AtYSL1 reveals its role in iron and nicotianamine seed loading. Plant J. 2005, 44, 769–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Chiecko, J.C.; Kim, S.A.; Walker, E.L.; Lee, Y.; Guerinot, M.L.; An, G. Disruption of OsYSL15 leads to iron inefficiency in rice plants. Plant Physiol. 2009, 150, 786–800. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ryoo, N.; Jeon, J.S.; Guerinot, M.L.; An, G. Activation of rice yellow stripe1-like 16 (OsYSL16) enhances iron efficiency. Mol. Cells 2012, 33, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, G.; Schikora, A.; Haberle, J.; Vert, G.; Ludewig, U.; Briat, J.F.; Curie, C.; von Wiren, N. A putative function for the Arabidopsis Fe-phytosiderophore transporter homolog AtYSL2 in Fe and Zn homeostasis. Plant Cell Physiol. 2005, 46, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Senoura, T.; Sakashita, E.; Kobayashi, T.; Takahashi, M.; Aung, M.S.; Masuda, H.; Nakanishi, H.; Nishizawa, N.K. The iron-chelate transporter OsYSL9 plays a role in iron distribution in developing rice grains. Plant Mol. Biol. 2017, 95, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Waters, B.M.; Chu, H.-H.; DiDonato, R.J.; Roberts, L.A.; Eisley, R.B.; Lahner, B.; Salt, D.E.; Walker, E.L. Mutations in Arabidopsis Yellow Stripe-Like1 and Yellow Stripe-Like3 reveal their roles in metal ion homeostasis and loading of metal ions in seeds. Plant Physiol. 2006, 141, 1446–1458. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yamaji, N.; Yokosho, K.; Ma, J.F. YSL16 is a phloem-localized transporter of the copper-nicotianamine complex that is responsible for copper distribution in rice. Plant Cell 2012, 24, 3767–3782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, W.; Yang, Y.; Shen, Z.; Ma, J.F.; Zheng, L. OsYSL16 is required for preferential Cu distribution to floral organs in rice. Plant Cell Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Yamaji, N.; Xia, J.; Ma, J.F. OsYSL6 is involved in the detoxification of excess manganese in rice. Plant Physiol. 2011, 157, 1832–1840. [Google Scholar] [CrossRef] [PubMed]
- Divol, F.; Couch, D.; Conejero, G.; Roschzttardtz, H.; Mari, S.; Curie, C. The Arabidopsis Yellow Stripe Like4 and 6 transporters control iron release from the chloroplast. Plant Cell 2013, 25, 1040–1055. [Google Scholar] [CrossRef] [PubMed]
- Conte, S.S.; Chu, H.H.; Chan-Rodriguez, D.; Punshon, T.; Vasques, K.A.; Salt, D.E.; Walker, E.L. Arabidopsis thalina Yellow Stripe1-Like4 and Yellow Stripe1-Like6 localize to internal cellular membranes and are involved in metal ion homeostasis. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Kobayashi, T.; Takahashi, M.; Nagasaka, S.; Usuda, K.; Kakei, Y.; Ishimaru, Y.; Nakanishi, H.; Mori, S.; Nishizawa, N.K. OsYSL18 is a rice iron(III)-deoxymugineic acid transporter specifically expressed in reproductive organs and phloem of lamina joints. Plant Mol. Biol. 2009, 70, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Itai, R.N.; Ogo, Y.; Kobayashi, T.; Nakanishi, H.; Nishizawa, N.K. Rice genes involved in phytosiderophore biosynthesis are synchronously regulated during the early stages of iron deficiency in roots. Rice 2013, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, C.; Wu, C.; Xiong, L.; Chen, G.; Zhang, Q.; Wang, S. RMD: A rice mutant database for functional analysis of the rice genome. Nucleic Acids Res. 2006, 34, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Itai, R.N.; Nishizawa, N.K. Iron deficiency responses in rice roots. Rice 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, Y.; Suzuki, M.; Tsukamoto, T.; Suzuki, K.; Nakazono, M.; Kobayashi, T.; Wada, Y.; Watanabe, S.; Matsuhashi, S.; Takahashi, M.; et al. Rice plants take up iron as an Fe3+ phytosiderophore and as Fe2+. Plant J. 2006, 45, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, Y.; Takahashi, R.; Bashir, K.; Shimo, H.; Senoura, T.; Sugimoto, K.; Ono, K.; Yano, M.; Ishikawa, S.; Arao, T.; et al. Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci. Rep. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.; Ishimaru, Y.; Shimo, H.; Nagasaka, S.; Fujimoto, M.; Takanashi, H.; Tsutsumi, N.; An, G.; Nakanishi, H.; Nishizawa, N.K. The rice mitochondrial iron transporter is essential for plant growth. Nat. Commun. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.H.; Yi, H.Y.; Gong, J.M. Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J. 2012, 72, 400–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokosho, K.; Yamaji, N.; Ueno, D.; Mitani, N.; Ma, J.F. OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiol. 2009, 149, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Yokosho, K.; Yamaji, N.; Ma, J.F. OsFRDL1 expressed in nodes is required for distribution of iron to grains in rice. J. Exp. Bot. 2016, 67, 5485–5494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoneyama, T.; Ishikawa, S.; Fujimaki, S. Route and Regulation of zinc, cadmium, and iron transport in rice plants (Oryza sativa L.) during vegetative growth and grain filling: Metal transporters, metal speciation, grain Cd reduction and Zn and Fe biofortification. Int. J. Mol. Sci. 2015, 16, 19111–19129. [Google Scholar] [CrossRef] [PubMed]
- Earley, K.W.; Haag, J.R.; Pontes, O.; Opper, K.; Juehne, T.; Song, K.M.; Pikaard, C.S. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 2006, 45, 616–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakagawa, T.; Kurose, T.; Hino, T.; Tanaka, K.; Kawamukai, M.; Niwa, Y.; Toyooka, K.; Matsuoka, K.; Jinbo, T.; Kimura, T. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 2007, 104, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Jin, W.Z.; Wang, M.Y.; Zhang, F.; Zhou, J.; Jia, O.J.; Wu, Y.R.; Liu, F.Y.; Wu, P. Distribution and characterization of over 1000 T-DNA tags in rice genome. Plant J. 2003, 36, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, K.Y.; Lim, S.H.; Kim, M.J.; Ye, S.J.; Lee, M.G.; Ha, S.H. Improvement of the fluorescence intensity during a flow cytometric analysis for rice protoplasts by localization of a green fluorescent protein into chloroplasts. Int. J. Mol. Sci. 2014, 16, 788–804. [Google Scholar] [CrossRef]
- Zhao, F.; McGrath, S.P.; Crosland, A.R. Comparison of 3 wet digestion methods for the determination of plant sulfur by inductively-coupled plasma-atomic emission-spectroscopy (ICPAES). Commun. Soil Sci. Plan. Anal. 1994, 25, 407–418. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Shinwari, K.I.; Luo, L.; Zheng, L. OsYSL13 Is Involved in Iron Distribution in Rice. Int. J. Mol. Sci. 2018, 19, 3537. https://doi.org/10.3390/ijms19113537
Zhang C, Shinwari KI, Luo L, Zheng L. OsYSL13 Is Involved in Iron Distribution in Rice. International Journal of Molecular Sciences. 2018; 19(11):3537. https://doi.org/10.3390/ijms19113537
Chicago/Turabian StyleZhang, Chang, Kamran Iqbal Shinwari, Le Luo, and Luqing Zheng. 2018. "OsYSL13 Is Involved in Iron Distribution in Rice" International Journal of Molecular Sciences 19, no. 11: 3537. https://doi.org/10.3390/ijms19113537
APA StyleZhang, C., Shinwari, K. I., Luo, L., & Zheng, L. (2018). OsYSL13 Is Involved in Iron Distribution in Rice. International Journal of Molecular Sciences, 19(11), 3537. https://doi.org/10.3390/ijms19113537