Blood‒Brain Barrier Pathology and CNS Outcomes in Streptococcus pneumoniae Meningitis
Abstract
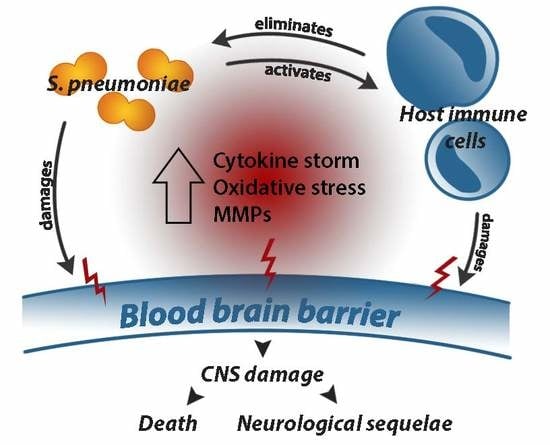
:1. Introduction
2. The BBB in Acute PM
2.1. Structure and Function of the BBB
2.2. Pneumococcal Transmigration across the BBB into the CNS
2.2.1. The Pneumococcal Capsule
2.2.2. Pneumococcal Proteins
2.2.3. Pneumolysin
2.3. Role of the Host Inflammatory Response in Determining Outcome in PM
2.3.1. Microglia and Immune Activation
2.3.2. Pattern Recognition Receptors
2.3.3. Leukocyte Infiltration and the Cytokine Storm
2.3.4. Reactive Oxygen and Nitrogen Species
2.3.5. Matrix Metalloproteinases
3. BBB Disruption and Long-Term Neurological Sequelae in PM
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APC | Antigen-presenting cells |
| ASC | apoptosis-associated speck-like protein containing a CARD domain |
| BBB | blood‒brain barrier |
| CASP1 | caspase-1 |
| Cbp | choline-binding proteins |
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| DAMP | danger-associated molecular patterns |
| HPA | hypothalamus‒pituitary‒adrenal |
| IFNγ | interferon-gamma |
| IL-1β | interleukin-1-beta |
| IL-6 | interleukin-6 |
| IL-12 | interleukin-12 |
| IL-18 | interleukin-18 |
| LytA | pneumococcal autolysin |
| MMP | matrix metalloproteinase |
| MyD88 | myeloid differentiation primary response 88 |
| NanA | neuraminidase A |
| NLR | Nod-like receptor |
| NLRP3 | Nod-like receptor (NLR) family pyrin domain containing 3 |
| NOS | nitric oxide synthase |
| PAF | platelet-activating factor |
| PAMP | pathogen-associated molecular patterns |
| PECAM-1 | platelet endothelial cell adhesion molecule |
| PLA2 | pneumococcal phospholipase 2 |
| plgR | poly immunoglobin receptor |
| ply | pneumolysin |
| PM | pneumococcal meningitis |
| PRR | pattern recognition receptors |
| Psp | pneumococcal surface proteins |
| PspA | pneumococcal surface protein A |
| PVM | perivascular macrophages |
| RONS | reactive oxygen and nitrogen species |
| ROS | reactive oxygen species |
| TACE | tumour necrosis factor alpha converting enzyme |
| TGF-β | transforming growth factor-beta |
| TLR | Toll-like receptor |
| TNF | tumour necrosis factor |
References
- Scarborough, M.; Thwaites, G.E. The diagnosis and management of acute bacterial meningitis in resource-poor settings. Lancet Neurol. 2008, 7, 637–648. [Google Scholar] [CrossRef]
- Watt, J.P.; Wolfson, L.J.; O’Brien, K.L.; Henkle, E.; Deloria-Knoll, M.; McCall, N.; Lee, E.; Levine, O.S.; Hajjeh, R.; Mulholland, K.; et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: Global estimates. Lancet 2009, 374, 903–911. [Google Scholar] [CrossRef]
- Scarborough, M.; Gordon, S.B.; Whitty, C.J.; French, N.; Njalale, Y.; Chitani, A.; Peto, T.E.; Lalloo, D.G.; Zijlstra, E.E. Corticosteroids for bacterial meningitis in adults in sub-Saharan Africa. N. Engl. J. Med. 2007, 357, 2441–2450. [Google Scholar] [CrossRef] [PubMed]
- Mook-Kanamori, B.B.; Geldhoff, M.; van der Poll, T.; van de Beek, D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin. Microbiol. Rev. 2011, 24, 557–591. [Google Scholar] [CrossRef] [PubMed]
- Schuchat, A.; Robinson, K.; Wenger, J.D.; Harrison, L.H.; Farley, M.; Reingold, A.L.; Lefkowitz, L.; Perkins, B.A. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N. Engl. J. Med. 1997, 337, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Gessner, B.D.; Mueller, J.E.; Yaro, S. African meningitis belt pneumococcal disease epidemiology indicates a need for an effective serotype 1 containing vaccine, including for older children and adults. BMC Infect. Dis. 2010, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Goetghebuer, T.; West, T.E.; Wermenbol, V.; Cadbury, A.L.; Milligan, P.; Lloyd-Evans, N.; Adegbola, R.A.; Mulholland, E.K.; Greenwood, B.M.; Weber, M.W. Outcome of meningitis caused by Streptococcus pneumoniae and Haemophilus influenzae type b in children in The Gambia. Trop. Med. Int. Health 2000, 5, 207–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, D.W.; Tenforde, M.W.; Mitchell, H.K.; Jarvis, J.N. Neurological sequelae of adult meningitis in Africa: A systematic literature review. Open Forum Infect. Dis. 2018, 5, ofx246. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, M.; Ulland, A.J.; Steinhardt, L.C.; Moïsi, J.C.; Were, F.; Levine, O.S. Sequelae due to bacterial meningitis among African children: A systematic literature review. BMC Med. 2009, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Christie, D.; Viner, R.M.; Knox, K.; Coen, P.G.; Wang, H.; El Bashir, H.; Legood, R.; Patel, B.C.; Booy, R. Long-term outcomes of pneumococcal meningitis in childhood and adolescence. Eur. J. Pediatr. 2011, 170, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Legood, R.; Coen, P.G.; Knox, K.; Viner, R.M.; El Bashir, H.; Christie, D.; Patel, B.C.; Booy, R. Health related quality of life in survivors of pneumococcal meningitis. Acta Paediatr. 2009, 98, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Whitney, C.G.; Farley, M.M.; Hadler, J.; Harrison, L.H.; Lexau, C.; Reingold, A.; Lefkowitz, L.; Cieslak, P.R.; Cetron, M.; Zell, E.R.; et al. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N. Engl. J. Med. 2000, 343, 1917–1924. [Google Scholar] [CrossRef] [PubMed]
- Leach, A.J.; Morris, P.S.; McCallum, G.B.; Wilson, C.A.; Stubbs, L.; Beissbarth, J.; Jacups, S.; Hare, K.; Smith-Vaughan, H.C. Emerging pneumococcal carriage serotypes in a high-risk population receiving universal 7-valent pneumococcal conjugate vaccine and 23-valent polysaccharide vaccine since 2001. BMC Infect. Dis. 2009, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Okade, H.; Funatsu, T.; Eto, M.; Furuya, Y.; Mizunaga, S.; Nomura, N.; Mitsuyama, J.; Yamagishi, Y.; Mikamo, H. Impact of the pneumococcal conjugate vaccine on serotype distribution and susceptibility trends of pediatric non-invasive Streptococcus pneumoniae isolates in Tokai, Japan over a 5-year period. J. Infect. Chemother. 2014, 20, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Pichon, B.; Ladhani, S.N.; Slack, M.P.; Segonds-Pichon, A.; Andrews, N.J.; Waight, P.A.; Miller, E.; George, R. Changes in molecular epidemiology of streptococcus pneumoniae causing meningitis following introduction of pneumococcal conjugate vaccination in England and Wales. J. Clin. Microbiol. 2013, 51, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Regev-Yochay, G.; Reisenberg, K.; Katzir, M.; Wiener-Well, Y.; Rahav, G.; Strahilevitz, J.; Istomin, V.; Tsyba, E.; Peretz, A.; Khakshoor, S.; et al. Pneumococcal Meningitis in Adults after Introduction of PCV7 and PCV13, Israel, July 2009–June 2015. Emerg. Infect. Dis. 2018, 24, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Antachopoulos, C.; Tsolia, M.N.; Tzanakaki, G.; Xirogianni, A.; Dedousi, O.; Markou, G.; Zografou, S.M.; Eliades, A.; Kirvassilis, F.; Kesanopoulos, K.; et al. Parapneumonic pleural effusions caused by Streptococcus pneumoniae serotype 3 in children immunised with 13-valent conjugated pneumococcal vaccine. Pediatr. Infect. Dis. J. 2014, 33, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Serlin, Y.; Shelef, I.; Knyazer, B.; Friedman, A. Anatomy and physiology of the blood-brain barrier. Semin. Cell Dev. Biol. 2015, 38, 2–6. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Mack, J.J.; Güç, E.; Warren, C.M.; Squadrito, M.L.; Kilarski, W.W.; Baer, C.; Freshman, R.D.; McDonald, A.I.; Ziyad, S.; et al. Perivascular macrophages limit permeability. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Herz, J.; Filiano, A.J.; Smith, A.; Yogev, N.; Kipnis, J. Myeloid Cells in the Central Nervous System. Immunity 2017, 46, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Sharif, Y.; Jumah, F.; Coplan, L.; Krosser, A.; Sharif, K.; Tubbs, R.S. The blood brain barrier: A review of its anatomy and physiology in health and disease. Clin. Anat. 2018. [Google Scholar] [CrossRef] [PubMed]
- Van Sorge, N.M.; Doran, K.S. Defense at the border: The blood-brain barrier versus bacterial foreigners. Future Microbiol. 2012, 7, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and dysfunction of the blood-brain barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef] [PubMed]
- Negi, N.; Das, B.K. CNS: Not an immunoprivilaged site anymore but a virtual secondary lymphoid organ. Int. Rev. Immunol. 2018, 37, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Harris, T.H.; Kipnis, J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol. 2015, 36, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, B.; Vajkoczy, P.; Weller, R.O. The movers and shapers in immune privilege of the CNS. Nat. Immunol. 2017, 18, 123. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, M.; Kipnis, J. A conceptual revolution in the relationships between the brain and immunity. Brain Behav. Immun. 2011, 25, 817–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, C.; Martin-Blondel, G.; Liblau, R.S. Endothelial cells and lymphatics at the interface between the immune and central nervous systems: Implications for multiple sclerosis. Curr. Opin. Neurol. 2017, 30, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.S.; Hunter, C.A. Protective and pathological immunity during central nervous system infections. Immunity 2017, 46, 891–909. [Google Scholar] [CrossRef] [PubMed]
- Pebody, R.G.; Morgan, O.; Choi, Y.; George, R.; Hussain, M.; Andrews, N. Use of antibiotics and risk factors for carriage of Streptococcus pneumoniae: A longitudinal household study in the United Kingdom. Epidemiol. Infect. 2009, 137, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Hammitt, L.L.; Bruden, D.L.; Butler, J.C.; Baggett, H.C.; Hurlburt, D.A.; Reasonover, A.; Hennessy, T.W. Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: An explanation of trends in invasive pneumococcal disease. J. Infect. Dis. 2006, 193, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Katsarolis, I.; Poulakou, G.; Analitis, A.; Matthaiopoulou, I.; Roilides, E.; Antachopoulos, C.; Kafetzis, D.A.; Daikos, G.L.; Vorou, R.; Koubaniou, C.; et al. Risk factors for nasopharyngeal carriage of drug-resistant Streptococcus pneumoniae: Data from a nation-wide surveillance study in Greece. BMC Infect. Dis. 2009, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Ardanuy, C.; Tubau, F.; Pallares, R.; Calatayud, L.; Domínguez, M.A.; Rolo, D.; Grau, I.; Martín, R.; Liñares, J. Epidemiology of invasive pneumococcal disease among adult patients in Barcelona before and after pediatric 7-valent pneumococcal conjugate vaccine introduction, 1997–-2007. Clin. Infect. Dis. 2009, 48, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Henriques-Normark, B.; Tuomanen, E.I. The pneumococcus: Epidemiology, microbiology, and pathogenesis. Cold Spring Harb. Perspect. Med. 2013, 3, a010215. [Google Scholar] [CrossRef] [PubMed]
- Weiser, J.N.; Ferreira, D.M.; Paton, J.C. Streptococcus pneumoniae: Transmission, colonization and invasion. Nat. Rev. Microbiol. 2018, 16, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Iovino, F.; Orihuela, C.J.; Moorlag, H.E.; Molema, G.; Bijlsma, J.J. Interactions between blood-borne Streptococcus pneumoniae and the blood-brain barrier preceding meningitis. PLoS ONE 2013, 8, e68408. [Google Scholar] [CrossRef] [PubMed]
- Van Ginkel, F.W.; McGhee, J.R.; Watt, J.M.; Campos-Torres, A.; Parish, L.A.; Briles, D.E. Pneumococcal carriage results in ganglioside-mediated olfactory tissue infection. Proc. Natl. Acad. Sci. USA 2003, 100, 14363–14367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doran, K.S.; Fulde, M.; Gratz, N.; Kim, B.J.; Nau, R.; Prasadarao, N.; Schubert-Unkmeir, A.; Tuomanen, E.I.; Valentin-Weigand, P. Host-pathogen interactions in bacterial meningitis. Acta Neuropathol. 2016, 131, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Magee, A.D.; Yother, J. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect. Immun. 2001, 69, 3755–3761. [Google Scholar] [CrossRef] [PubMed]
- Kadioglu, A.; Weiser, J.N.; Paton, J.C.; Andrew, P.W. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat. Rev. Microbiol. 2008, 6, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Wartha, F.; Beiter, K.; Albiger, B.; Fernebro, J.; Zychlinsky, A.; Normark, S.; Henriques-Normark, B. Capsule and D-alanylated lipoteichoic acids protect Streptococcus pneumoniae against neutrophil extracellular traps. Cell. Microbiol. 2007, 9, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Middleton, D.R.; Paschall, A.V.; Duke, J.A.; Avci, F.Y. Enzymatic hydrolysis of pneumococcal capsular polysaccharide renders the bacterium vulnerable to host defense. Infect. Immun. 2018. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.M.; Mitchell, T.J. Streptococcus pneumoniae: Virulence factors and variation. Clin. Microbiol. Infect. 2010, 16, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.E.; Jones, C.V.; Thornton, J.A.; Sanders, M.E.; Swiatlo, E.; Nahm, M.H.; Park, I.H.; McDaniel, L.S. PspK of Streptococcus pneumoniae increases adherence to epithelial cells and enhances nasopharyngeal colonization. Infect. Immun. 2013, 81, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Li-Korotky, H.S.; Lo, C.Y.; Banks, J.M. Interaction of pneumococcal phase variation, host and pressure/gas composition: Virulence expression of NanA, HylA, PspA and CbpA in simulated otitis media. Microb. Pathog. 2010, 49, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Shainheit, M.G.; Mule, M.; Camilli, A. The core promoter of the capsule operon of Streptococcus pneumoniae is necessary for colonization and invasive disease. Infect. Immun. 2014, 82, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Bagnoli, F.; Moschioni, M.; Donati, C.; Dimitrovska, V.; Ferlenghi, I.; Facciotti, C.; Muzzi, A.; Giusti, F.; Emolo, C.; Sinisi, A.; et al. A second pilus type in Streptococcus pneumoniae is prevalent in emerging serotypes and mediates adhesion to host cells. J. Bacteriol. 2008, 190, 5480–5492. [Google Scholar] [CrossRef] [PubMed]
- Iovino, F.; Hammarlöf, D.L.; Garriss, G.; Brovall, S.; Nannapaneni, P.; Henriques-Normark, B. Pneumococcal meningitis is promoted by single cocci expressing pilus adhesin RrgA. J. Clin. Investig. 2016, 126, 2821–2826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iovino, F.; Engelen-Lee, J.Y.; Brouwer, M.; van de Beek, D.; van der Ende, A.; Valls Seron, M.; Mellroth, P.; Muschiol, S.; Bergstrand, J.; Widengren, J.; et al. pIgR and PECAM-1 bind to pneumococcal adhesins RrgA and PspC mediating bacterial brain invasion. J. Exp. Med. 2017, 214, 1619–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchiyama, S.; Carlin, A.F.; Khosravi, A.; Weiman, S.; Banerjee, A.; Quach, D.; Hightower, G.; Mitchell, T.J.; Doran, K.S.; Nizet, V. The surface-anchored NanA protein promotes pneumococcal brain endothelial cell invasion. J. Exp. Med. 2009, 206, 1845–1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neeleman, C.; Geelen, S.P.; Aerts, P.C.; Daha, M.R.; Mollnes, T.E.; Roord, J.J.; Posthuma, G.; van Dijk, H.; Fleer, A. Resistance to both complement activation and phagocytosis in type 3 pneumococci is mediated by the binding of complement regulatory protein factor H. Infect. Immun. 1999, 67, 4517–4524. [Google Scholar] [PubMed]
- Smith, B.L.; Hostetter, M.K. C3 as substrate for adhesion of Streptococcus pneumoniae. J. Infect. Dis. 2000, 182, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Van der Maten, E.; Westra, D.; van Selm, S.; Langereis, J.D.; Bootsma, H.J.; van Opzeeland, F.J.; de Groot, R.; Ruseva, M.M.; Pickering, M.C.; van den Heuvel, L.P.; et al. Complement Factor H serum levels determine resistance to pneumococcal invasive disease. J. Infect. Dis. 2016, 213, 1820–1827. [Google Scholar] [CrossRef] [PubMed]
- Mirza, S.; Benjamin, W.H., Jr.; Coan, P.A.; Hwang, S.A.; Winslett, A.K.; Yother, J.; Hollingshead, S.K.; Fujihashi, K.; Briles, D.E. The effects of differences in pspA alleles and capsular types on the resistance of Streptococcus pneumoniae to killing by apolactoferrin. Microb. Pathog. 2016, 99, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Andre, G.O.; Politano, W.R.; Mirza, S.; Converso, T.R.; Ferraz, L.F.; Leite, L.C.; Darrieux, M. Combined effects of lactoferrin and lysozyme on Streptococcus pneumoniae killing. Microb. Pathog. 2015, 89, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Dave, S.; Carmicle, S.; Hammerschmidt, S.; Pangburn, M.K.; McDaniel, L.S. Dual roles of PspC, a surface protein of Streptococcus pneumoniae, in binding human secretory IgA and factor H. J. Immunol. 2004, 173, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, S.; Tillig, M.P.; Wolff, S.; Vaerman, J.P.; Chhatwal, G.S. Species-specific binding of human secretory component to SpsA protein of Streptococcus pneumoniae via a hexapeptide motif. Mol. Microbiol. 2000, 36, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, C.J.; Gao, G.; Francis, K.P.; Yu, J.; Tuomanen, E.I. Tissue-specific contributions of pneumococcal virulence factors to pathogenesis. J. Infect. Dis. 2004, 190, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Yuste, J.; Botto, M.; Paton, J.C.; Holden, D.W.; Brown, J.S. Additive inhibition of complement deposition by pneumolysin and PspA facilitates Streptococcus pneumoniae septicemia. J. Immunol. 2005, 175, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Rosenow, C.; Ryan, P.; Weiser, J.N.; Johnson, S.; Fontan, P.; Ortqvist, A.; Masure, H.R. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol. Microbiol. 1997, 25, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Sitkiewicz, I.; Stockbauer, K.E.; Musser, J.M. Secreted bacterial phospholipase A2 enzymes: Better living through phospholipolysis. Trends Microbiol. 2007, 15, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Cremers, A.J.H.; Mobegi, F.M.; van der Gaast-de Jongh, C.; van Weert, M.; van Opzeeland, F.J.; Vehkala, M.; Knol, M.J.; Bootsma, H.J.; Välimäki, N.; Croucher, N.J.; et al. The contribution of genetic variation of Streptococcus pneumoniae to the clinical manifestation of invasive pneumococcal disease. Clin. Infect. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Domon, H.; Kurosawa, M.; Isono, T.; Maekawa, T.; Yamaguchi, M.; Kawabata, S.; Terao, Y. Streptococcus pyogenes Phospholipase A2 induces the expression of adhesion molecules on human umbilical vein endothelial cells and aorta of mice. Front. Cell. Infect. Microbiol. 2017, 7, 300. [Google Scholar] [CrossRef] [PubMed]
- Tuomanen, E.; Liu, H.; Hengstler, B.; Zak, O.; Tomasz, A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J. Infect. Dis. 1985, 151, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Wren, J.T.; Blevins, L.K.; Pang, B.; Roy, A.B.; Oliver, M.B.; Reimche, J.L.; Wozniak, J.E.; Alexander-Miller, M.A.; Swords, W.E. Pneumococcal Neuraminidase A (NanA) promotes biofilm formation and synergizes with Influenza A virus in nasal colonization and middle ear infection. Infect. Immun. 2017, 85, e01044-16. [Google Scholar] [CrossRef] [PubMed]
- Janoff, E.N.; Rubins, J.B.; Fasching, C.; Charboneau, D.; Rahkola, J.T.; Plaut, A.G.; Weiser, J.N. Pneumococcal IgA1 protease subverts specific protection by human IgA1. Mucosal Immunol. 2014, 7, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Suits, M.D.; Pluvinage, B.; Law, A.; Liu, Y.; Palma, A.S.; Chai, W.; Feizi, T.; Boraston, A.B. Conformational analysis of the Streptococcus pneumoniae hyaluronate lyase and characterization of its hyaluronan-specific carbohydrate-binding module. J. Biol. Chem. 2014, 289, 27264–27277. [Google Scholar] [CrossRef] [PubMed]
- Berry, A.M.; Paton, J.C. Additive attenuation of virulence of Streptococcus pneumoniae by mutation of the genes encoding pneumolysin and other putative pneumococcal virulence proteins. Infect. Immun. 2000, 68, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Pericone, C.D.; Overweg, K.; Hermans, P.W.; Weiser, J.N. Inhibitory and bactericidal effects of hydrogen peroxide production by Streptococcus pneumoniae on other inhabitants of the upper respiratory tract. Infect. Immun. 2000, 68, 3990–3997. [Google Scholar] [CrossRef] [PubMed]
- Paton, J.C.; Andrew, P.W.; Boulnois, G.J.; Mitchell, T.J. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: The role of pneumococcal proteins. Annu. Rev. Microbiol. 1993, 47, 89–115. [Google Scholar] [CrossRef] [PubMed]
- Price, K.E.; Greene, N.G.; Camilli, A. Export requirements of pneumolysin in Streptococcus pneumoniae. J. Bacteriol. 2012, 194, 3651–3660. [Google Scholar] [CrossRef] [PubMed]
- van Pee, K.; Mulvihill, E.; Müller, D.J.; Yildiz, Ö. Unraveling the pore-forming steps of pneumolysin from Streptococcus pneumoniae. Nano Lett. 2016, 16, 7915–7924. [Google Scholar] [CrossRef] [PubMed]
- Marriott, H.M.; Mitchell, T.J.; Dockrell, D.H. Pneumolysin: A double-edged sword during the host-pathogen interaction. Curr. Mol. Med. 2008, 8, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Domon, H.; Maekawa, T.; Oda, M.; Hiyoshi, T.; Tamura, H.; Yonezawa, D.; Arai, Y.; Yokoji, M.; Tabeta, K. Pneumococcal DNA-binding proteins released through autolysis induce the production of proinflammatory cytokines via toll-like receptor 4. Cell. Immunol. 2018, 325, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Witzenrath, M.; Pache, F.; Lorenz, D.; Koppe, U.; Gutbier, B.; Tabeling, C.; Reppe, K.; Meixenberger, K.; Dorhoi, A.; Ma, J.; et al. The NLRP3 inflammasome is differentially activated by pneumolysin variants and contributes to host defense in pneumococcal pneumonia. J. Immunol. 2011, 187, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Martner, A.; Dahlgren, C.; Paton, J.C.; Wold, A.E. Pneumolysin released during Streptococcus pneumoniae autolysis is a potent activator of intracellular oxygen radical production in neutrophils. Infect. Immun. 2008, 76, 4079–4087. [Google Scholar] [CrossRef] [PubMed]
- Hirst, R.A.; Rutman, A.; Sikand, K.; Andrew, P.W.; Mitchell, T.J.; O’Callaghan, C. Effect of pneumolysin on rat brain ciliary function: Comparison of brain slices with cultured ependymal cells. Pediatr. Res. 2000, 47, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Hirst, R.A.; Gosai, B.; Rutman, A.; Andrew, P.W.; O’Callaghan, C. Streptococcus pneumoniae damages the ciliated ependyma of the brain during meningitis. Infect. Immun. 2003, 71, 6095–6100. [Google Scholar] [CrossRef] [PubMed]
- Zysk, G.; Schneider-Wald, B.K.; Hwang, J.H.; Bejo, L.; Kim, K.S.; Mitchell, T.J.; Hakenbeck, R.; Heinz, H.P. Pneumolysin is the main inducer of cytotoxicity to brain microvascular endothelial cells caused by Streptococcus pneumoniae. Infect. Immun. 2001, 69, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.S.; Hoffmann, O.; Schickhaus, M.; Freyer, D.; Dagand, E.; Bermpohl, D.; Mitchell, T.J.; Bechmann, I.; Weber, J.R. Pneumolysin causes neuronal cell death through mitochondrial damage. Infect. Immun. 2007, 75, 4245–4254. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Paton, J.C.; Briles, D.E.; Rhee, D.K.; Pyo, S. Streptococcus pneumoniae induces pyroptosis through the regulation of autophagy in murine microglia. Oncotarget 2015, 6, 44161–44178. [Google Scholar] [CrossRef] [PubMed]
- Hupp, S.; Heimeroth, V.; Wippel, C.; Förtsch, C.; Ma, J.; Mitchell, T.J.; Iliev, A.I. Astrocytic tissue remodeling by the meningitis neurotoxin pneumolysin facilitates pathogen tissue penetration and produces interstitial brain edema. Glia 2012, 60, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Wall, E.C.; Gordon, S.B.; Hussain, S.; Goonetilleke, U.R.; Gritzfeld, J.; Scarborough, M.; Kadioglu, A. Persistence of pneumolysin in the cerebrospinal fluid of patients with pneumococcal meningitis is associated with mortality. Clin. Infect. Dis. 2012, 54, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Hirst, R.A.; Gosai, B.; Rutman, A.; Guerin, C.J.; Nicotera, P.; Andrew, P.W.; O’Callaghan, C. Streptococcus pneumoniae deficient in pneumolysin or autolysin has reduced virulence in meningitis. J. Infect. Dis. 2008, 197, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Yau, B. Pathogenesis of pneumococcal meningitis. Ph.D. Thesis, University of Sydney, Sydney, Australia, 2014; pp. 118–121. [Google Scholar]
- Yamasaki, R.; Lu, H.; Butovsky, O.; Ohno, N.; Rietsch, A.M.; Cialic, R.; Wu, P.M.; Doykan, C.E.; Lin, J.; Cotleur, A.C.; et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 2014, 211, 1533–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barichello, T.; Generoso, J.S.; Simões, L.R.; Goularte, J.A.; Petronilho, F.; Saigal, P.; Badawy, M.; Quevedo, J. Role of microglial activation in the pathophysiology of bacterial meningitis. Mol. Neurobiol. 2016, 53, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Ribes, S.; Ebert, S.; Regen, T.; Agarwal, A.; Tauber, S.C.; Czesnik, D.; Spreer, A.; Bunkowski, S.; Eiffert, H.; Hanisch, U.K.; et al. Toll-like receptor stimulation enhances phagocytosis and intracellular killing of nonencapsulated and encapsulated Streptococcus pneumoniae by murine microglia. Infect. Immun. 2010, 78, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Peppoloni, S.; Colombari, B.; Beninati, C.; Felici, F.; Teti, G.; Speziale, P.; Ricci, S.; Ardizzoni, A.; Manca, L.; Blasi, E. The Spr1875 protein confers resistance to the microglia-mediated killing of Streptococcus pneumoniae. Microb. Pathog. 2013, 59–60, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Shaked, I.; Porat, Z.; Gersner, R.; Kipnis, J.; Schwartz, M. Early activation of microglia as antigen-presenting cells correlates with T cell-mediated protection and repair of the injured central nervous system. J. Neuroimmunol. 2004, 146, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, L.O.; Varoga, D.; Nicolaeva, N.; Leib, S.L.; Wilms, H.; Podschun, R.; Wruck, C.J.; Schröder, J.M.; Pufe, T.; Lucius, R. Role of glial cells in the functional expression of LL-37/rat cathelin-related antimicrobial peptide in meningitis. J. Neuropathol. Exp. Neurol. 2008, 67, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Vijay, K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int. Immunopharmacol. 2018, 59, 391–412. [Google Scholar] [CrossRef] [PubMed]
- Koppe, U.; Högner, K.; Doehn, J.M.; Müller, H.C.; Witzenrath, M.; Gutbier, B.; Bauer, S.; Pribyl, T.; Hammerschmidt, S.; Lohmeyer, J.; et al. Streptococcus pneumoniae stimulates a STING- and IFN regulatory factor 3-dependent type I IFN production in macrophages, which regulates RANTES production in macrophages, cocultured alveolar epithelial cells, and mouse lungs. J. Immunol. 2012, 188, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Lien, E.; Ingalls, R.R.; Tuomanen, E.; Dziarski, R.; Golenbock, D. Cutting edge: Recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 1999, 163, 1–5. [Google Scholar] [PubMed]
- Schroder, N.W.; Morath, S.; Alexander, C.; Hamann, L.; Hartung, T.; Zähringer, U.; Göbel, U.B.; Weber, J.R.; Schumann, R.R. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J. Biol. Chem. 2003, 278, 15587–15594. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, G.; Chimalapati, S.; Pollard, T.; Lapp, T.; Cohen, J.; Camberlein, E.; Stafford, S.; Periselneris, J.; Aldridge, C.; Vollmer, W.; et al. TLR-mediated inflammatory responses to Streptococcus pneumoniae are highly dependent on surface expression of bacterial lipoproteins. J. Immunol. 2014, 193, 3736–3745. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sierra, S.; Golenbock, D.T.; Henneke, P. Toll-like receptor-dependent discrimination of streptococci. J. Endotoxin Res. 2006, 12, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H.; Paludan, S.R.; Kilian, M.; Ostergaard, L. Live Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis activate the inflammatory response through Toll-like receptors 2, 4, and 9 in species-specific patterns. J. Leukocyte Biol. 2006, 80, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Leifer, C.A.; Kennedy, M.N.; Mazzoni, A.; Lee, C.; Kruhlak, M.J.; Segal, D.M. TLR9 is localized in the endoplasmic reticulum prior to stimulation. J. Immunol. 2004, 173, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.G.; Muruve, D.A.; Power, C. Inflammasomes in the CNS. Nat. Rev. Neurosci. 2014, 15, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Rabes, A.; Suttorp, N.; Opitz, B. Inflammasomes in pneumococcal Infection: Innate immune sensing and bacterial evasion strategies. Curr. Top. Microbiol. Immunol. 2016, 397, 215–227. [Google Scholar] [PubMed]
- McNeela, E.A.; Burke, A.; Neill, D.R.; Baxter, C.; Fernandes, V.E.; Ferreira, D.; Smeaton, S.; El-Rachkidy, R.; McLoughlin, R.M.; Mori, A.; et al. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog. 2010, 6, e1001191. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Zhou, R.; Tschopp, J. The NLRP3 inflammasome: A sensor for metabolic danger? Science 2010, 327, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Hoegen, T.; Tremel, N.; Klein, M.; Angele, B.; Wagner, H.; Kirschning, C.; Pfister, H.W.; Fontana, A.; Hammerschmidt, S.; Koedel, U. The NLRP3 inflammasome contributes to brain injury in pneumococcal meningitis and is activated through ATP-dependent lysosomal cathepsin B release. J. Immunol. 2011, 187, 5440–5451. [Google Scholar] [CrossRef] [PubMed]
- Fang, R.; Hara, H.; Sakai, S.; Hernandez-Cuellar, E.; Mitsuyama, M.; Kawamura, I.; Tsuchiya, K. Type I interferon signaling regulates activation of the absent in melanoma 2 inflammasome during Streptococcus pneumoniae infection. Infect. Immun. 2014, 82, 2310–2317. [Google Scholar] [CrossRef] [PubMed]
- Sollberger, G.; Strittmatter, G.E.; Garstkiewicz, M.; Sand, J.; Beer, H.D. Caspase-1: The inflammasome and beyond. Innate Immun. 2014, 20, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Geldhoff, M.; Mook-Kanamori, B.B.; Brouwer, M.C.; Troost, D.; Leemans, J.C.; Flavell, R.A.; Van der Ende, A.; Van der Poll, T.; Van de Beek, D. Inflammasome activation mediates inflammation and outcome in humans and mice with pneumococcal meningitis. BMC Infect. Dis. 2013, 13, 358. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Yau, B.; McQuillan, J.A.; Ball, H.J.; Too, L.K.; Abtin, A.; Hertzog, P.; Leib, S.L.; Jones, C.A.; Gerega, S.K.; et al. Inflammasome-dependent IFN-gamma drives pathogenesis in Streptococcus pneumoniae meningitis. J. Immunol. 2012, 189, 4970–4980. [Google Scholar] [CrossRef] [PubMed]
- Ferwerda, B.; Valls Serón, M.; Jongejan, A.; Zwinderman, A.H.; Geldhoff, M.; van der Ende, A.; Baas, F.; Brouwer, M.C.; van de Beek, D. Variation of 46 Innate Immune genes evaluated for their contribution in pneumococcal meningitis susceptibility and outcome. eBioMedicine 2016, 10, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Wall, E.C.; Gritzfeld, J.F.; Scarborough, M.; Ajdukiewicz, K.M.; Mukaka, M.; Corless, C.; Lalloo, D.G.; Gordon, S.B. Genomic pneumococcal load and CSF cytokines are not related to outcome in Malawian adults with meningitis. J. Infect. 2014, 69, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, F.; Parizzi, F.; Garlaschi, L.; Assael, B.M.; Sironi, M.; Ghezzi, P.; Mantovani, A. Interleukin 6 activity in infants and children with bacterial meningitis. The Collaborative Study on Meningitis. Pediatr. Infect. Dis. J. 1991, 10, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, L.G.; Grandgirard, D.; Leib, S.L.; Agnez-Lima, L.F. Cerebrospinal-fluid cytokine and chemokine profile in patients with pneumococcal and meningococcal meningitis. BMC Infect. Dis. 2013, 13, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diab, A.; Zhu, J.; Lindquist, L.; Wretlind, B.; Bakhiet, M.; Link, H. Haemophilus influenzae and Streptococcus pneumoniae induce different intracerebral mRNA cytokine patterns during the course of experimental bacterial meningitis. Clin. Exp. Immunol. 1997, 109, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Mook-Kanamori, B.; Geldhoff, M.; Troost, D.; van der Poll, T.; van de Beek, D. Characterization of a pneumococcal meningitis mouse model. BMC Infect. Dis. 2012, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lian, D.; Wu, J.; Liu, Y.; Zhu, M.; Sun, J.; He, D.; Li, L. Brain-derived neurotrophic factor reduces inflammation and hippocampal apoptosis in experimental Streptococcus pneumoniae meningitis. J. Neuroinflamm. 2017, 14, 156. [Google Scholar] [CrossRef] [PubMed]
- Yau, B.; Too, L.K.; Ball, H.J.; Hunt, N.H. TIGR4 strain causes more severe disease than WU2 strain in a mouse model of Streptococcus pneumoniae meningitis: A common pathogenic role for interferon-gamma. Microbes Infect. 2017, 19, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Polfliet, M.M.; Zwijnenburg, P.J.; van Furth, A.M.; van der Poll, T.; Döpp, E.A.; Renardel de Lavalette, C.; van Kesteren-Hendrikx, E.M.; van Rooijen, N.; Dijkstra, C.D.; van den Berg, T.K. Meningeal and perivascular macrophages of the central nervous system play a protective role during bacterial meningitis. J. Immunol. 2001, 167, 4644–4650. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; MacLean, A.G.; Philipp, M.T. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediat. Inflamm. 2013, 2013, 480739. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, Y.; Ransohoff, R.M. Inflammatory cell trafficking across the blood-brain barrier: Chemokine regulation and in vitro models. Immunol. Rev. 2012, 248, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Man, S.; Tucky, B.; Cotleur, A.; Drazba, J.; Takeshita, Y.; Ransohoff, R.M. CXCL12-induced monocyte-endothelial interactions promote lymphocyte transmigration across an in vitro blood-brain barrier. Sci. Transl. Med. 2012, 4, 119ra14. [Google Scholar] [CrossRef] [PubMed]
- Carman, C.V. Mechanisms for transcellular diapedesis: Probing and pathfinding by ‘invadosome-like protrusions’. J. Cell Sci. 2009, 122, 3025–3035. [Google Scholar] [CrossRef] [PubMed]
- Wewer, C.; Seibt, A.; Wolburg, H.; Greune, L.; Schmidt, M.A.; Berger, J.; Galla, H.J.; Quitsch, U.; Schwerk, C.; Schroten, H.; et al. Transcellular migration of neutrophil granulocytes through the blood-cerebrospinal fluid barrier after infection with Streptococcus suis. J. Neuroinflamm. 2011, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Kornelisse, R.F.; Westerbeek, C.M.; Spoor, A.B.; van der Heijde, B.; Spanjaard, L.; Neijens, H.J.; de Groot, R. Pneumococcal meningitis in children: Prognostic indicators and outcome. Clin. Infect. Dis. 1995, 21, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Brandt, C.T. Experimental studies of pneumococcal meningitis. Dan. Med. Bull. 2010, 57, B4119. [Google Scholar] [PubMed]
- Yau, B. Pathogenesis of Pneumococcal Meningitis. Ph.D. Thesis, University of Sydney, Sydney, Australia, 2014; pp. 174–175. [Google Scholar]
- Koedel, U.; Frankenberg, T.; Kirschnek, S.; Obermaier, B.; Häcker, H.; Paul, R.; Häcker, G. Apoptosis is essential for neutrophil functional shutdown and determines tissue damage in experimental pneumococcal meningitis. PLoS Pathog. 2009, 5, e1000461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Too, L.K.; Mitchell, A.J.; McGregor, I.S.; Hunt, N.H. Antibody-induced neutrophil depletion prior to the onset of pneumococcal meningitis influences long-term neurological complications in mice. Brain Behav. Immun. 2016, 56, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Collodel, A.; Generoso, J.S.; Simões, L.R.; Moreira, A.P.; Ceretta, R.A.; Petronilho, F.; Quevedo, J. Targets for adjunctive therapy in pneumococcal meningitis. J. Neuroimmunol. 2015, 278, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; dos Santos, I.; Savi, G.D.; Simões, L.R.; Silvestre, T.; Comim, C.M.; Sachs, D.; Teixeira, M.M.; Teixeira, A.L.; Quevedo, J. TNF-alpha, IL-1beta, IL-6, and cinc-1 levels in rat brain after meningitis induced by Streptococcus pneumoniae. J. Neuroimmunol. 2010, 221, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Yau, B.; Mitchell, A.J.; Too, L.K.; Ball, H.J.; Hunt, N.H. Interferon-gamma-induced nitric oxide synthase-2 contributes to blood/brain barrier dysfunction and acute mortality in experimental Streptococcus pneumoniae meningitis. J. Interferon Cytokine Res. 2016, 36, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Grandgirard, D.; Gäumann, R.; Coulibaly, B.; Dangy, J.P.; Sie, A.; Junghanss, T.; Schudel, H.; Pluschke, G.; Leib, S.L. The causative pathogen determines the inflammatory profile in cerebrospinal fluid and outcome in patients with bacterial meningitis. Mediat. Inflamm. 2013, 2013, 312476. [Google Scholar] [CrossRef] [PubMed]
- Quagliarello, V.J.; Wispelwey, B.; Long, W.J., Jr.; Scheld, W.M. Recombinant human interleukin-1 induces meningitis and blood-brain barrier injury in the rat. Characterization and comparison with tumor necrosis factor. J. Clin. Investig. 1991, 87, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Wellmer, A.; Gerber, J.; Ragheb, J.; Zysk, G.; Kunst, T.; Smirnov, A.; Brück, W.; Nau, R. Effect of deficiency of tumor necrosis factor alpha or both of its receptors on Streptococcus pneumoniae central nervous system infection and peritonitis. Infect. Immun. 2001, 69, 6881–6886. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, L.J.; Tauber, S.C.; Merres, J.; Kress, E.; Stope, M.B.; Jansen, S.; Pufe, T.; Brandenburg, L.O. Lack of proinflammatory cytokine interleukin-6 or tumor necrosis factor receptor-1 results in a failure of the innate immune response after bacterial meningitis. Mediat. Inflamm. 2016, 2016, 7678542. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Koedel, U.; Winkler, F.; Kieseier, B.C.; Fontana, A.; Kopf, M.; Hartung, H.P.; Pfister, H.W. Lack of IL-6 augments inflammatory response but decreases vascular permeability in bacterial meningitis. Brain 2003, 126, 1873–1882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koedel, U.; Winkler, F.; Angele, B.; Fontana, A.; Flavell, R.A.; Pfister, H.W. Role of Caspase-1 in experimental pneumococcal meningitis: Evidence from pharmacologic Caspase inhibition and Caspase-1-deficient mice. Ann. Neurol. 2002, 51, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Zwijnenburg, P.J.; van der Poll, T.; Florquin, S.; Roord, J.J.; Van Furth, A.M. IL-1 receptor type 1 gene-deficient mice demonstrate an impaired host defense against pneumococcal meningitis. J. Immunol. 2003, 170, 4724–4730. [Google Scholar] [CrossRef] [PubMed]
- Kornelisse, R.F.; Hack, C.E.; Savelkoul, H.F.; van der Pouw Kraan, T.C.; Hop, W.C.; van Mierlo, G.; Suur, M.H.; Neijens, H.J.; de Groot, R. Intrathecal production of interleukin-12 and gamma interferon in patients with bacterial meningitis. Infect. Immun. 1997, 65, 877–881. [Google Scholar] [PubMed]
- Too, L.K.; Ball, H.J.; McGregor, I.S.; Hunt, N.H. The pro-inflammatory cytokine interferon-gamma is an important driver of neuropathology and behavioural sequelae in experimental pneumococcal meningitis. Brain Behav. Immun. 2014, 40, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Pettini, E.; Fiorino, F.; Cuppone, A.M.; Iannelli, F.; Medaglini, D.; Pozzi, G. Interferon-gamma from brain leukocytes enhances meningitis by type 4 Streptococcus pneumoniae. Front. Microbiol. 2015, 6, 1340. [Google Scholar] [CrossRef] [PubMed]
- Hausler, K.G.; Prinz, M.; Nolte, C.; Weber, J.R.; Schumann, R.R.; Kettenmann, H.; Hanisch, U.K. Interferon-gamma differentially modulates the release of cytokines and chemokines in lipopolysaccharide- and pneumococcal cell wall-stimulated mouse microglia and macrophages. Eur. J. Neurosci. 2002, 16, 2113–2122. [Google Scholar] [CrossRef] [PubMed]
- Okamura, H.; Kashiwamura, S.; Tsutsui, H.; Yoshimoto, T.; Nakanishi, K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr. Opin. Immunol. 1998, 10, 259–264. [Google Scholar] [CrossRef]
- Barichello, T.; Generoso, J.S.; Simões, L.R.; Elias, S.G.; Quevedo, J. Role of oxidative stress in the pathophysiology of pneumococcal meningitis. Oxid. Med. Cell. Longev. 2013, 2013, 371465. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Koedel, U.; Pfister, H.W. Oxidative stress in pneumococcal meningitis: A future target for adjunctive therapy? Prog. Neurobiol. 2006, 80, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Schaper, M.; Leib, S.L.; Meli, D.N.; Brandes, R.P.; Täuber, M.G.; Christen, S. Differential effect of p47phox and gp91phox deficiency on the course of Pneumococcal Meningitis. Infect. Immun. 2003, 71, 4087–4092. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Lemos, J.C.; Generoso, J.S.; Cipriano, A.L.; Milioli, G.L.; Marcelino, D.M.; Vuolo, F.; Petronilho, F.; Dal-Pizzol, F.; Vilela, M.C. Oxidative stress, cytokine/chemokine and disruption of blood-brain barrier in neonate rats after meningitis by Streptococcus agalactiae. Neurochem. Res. 2011, 36, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Parrish, M.; Tay, I.J.; Li, N.; Ackerman, S.; He, F.; Kwang, J.; Chow, V.T. Engelward BP4. Streptococcus pneumoniae secretes hydrogen peroxide leading to DNA damage and apoptosis in lung cells. Proc. Natl. Acad. Sci. USA 2015, 112, E3421–E3430. [Google Scholar] [CrossRef] [PubMed]
- Kastenbauer, S.; Koedel, U.; Pfister, H.W. Role of peroxynitrite as a mediator of pathophysiological alterations in experimental pneumococcal meningitis. J. Infect. Dis. 1999, 180, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Meli, D.N.; Christen, S.; Leib, S.L. Matrix metalloproteinase-9 in pneumococcal meningitis: Activation via an oxidative pathway. J. Infect. Dis. 2003, 187, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Pfister, H.W.; Koedel, U.; Dirnagl, U.; Haberl, R.L.; Feiden, W.; Einhäupl, K.M. Superoxide dismutase inhibits brain oedema formation in experimental pneumococcal meningitis. Acta Neurochir. Suppl. 1990, 51, 378–380. [Google Scholar] [PubMed]
- Pfister, H.W.; Koedel, U.; Lorenzl, S.; Tomasz, A. Antioxidants attenuate microvascular changes in the early phase of experimental pneumococcal meningitis in rats. Stroke 1992, 23, 1798–1804. [Google Scholar] [CrossRef] [PubMed]
- Kastenbauer, S.; Koedel, U.; Becker, B.F.; Pfister, H.W. Oxidative stress in bacterial meningitis in humans. Neurology 2002, 58, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Winkler, F.; Koedel, U.; Kastenbauer, S.; Pfister, H.W. Differential expression of nitric oxide synthases in bacterial meningitis: Role of the inducible isoform for blood-brain barrier breakdown. J. Infect. Dis. 2001, 183, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.S.; Novak, R.; Herzog, K.H.; Bodner, S.M.; Cleveland, J.L.; Tuomanen, E.I. Neuroprotection by a caspase inhibitor in acute bacterial meningitis. Nat. Med. 1999, 5, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Koedel, U.; Paul, R.; Winkler, F.; Kastenbauer, S.; Huang, P.L.; Pfister, H.W. Lack of endothelial nitric oxide synthase aggravates murine pneumococcal meningitis. J. Neuropathol. Exp. Neurol. 2001, 60, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ma, Q.Q.; Yan, Y.; Xu, F.D.; Zhang, X.Y.; Zhou, W.Q.; Feng, Z.C. Edaravone attenuates hippocampal damage in an infant mouse model of pneumococcal meningitis by reducing HMGB1 and iNOS expression via the Nrf2/HO-1 pathway. Acta Pharmacol. Sin. 2016, 37, 1298–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konnecke, H.; Bechmann, I. The role of microglia and matrix metalloproteinases involvement in neuroinflammation and gliomas. Clin. Dev. Immunol. 2013, 2013, 914104. [Google Scholar] [CrossRef] [PubMed]
- Leib, S.L.; Leppert, D.; Clements, J.; Täuber, M.G. Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect. Immun. 2000, 68, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Leppert, D.; Leib, S.L.; Grygar, C.; Miller, K.M.; Schaad, U.B.; Holländer, G.A. Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: Association with blood-brain barrier damage and neurological sequelae. Clin. Infect. Dis. 2000, 31, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Liechti, F.D.; Grandgirard, D.; Leppert, D.; Leib, S.L. Matrix metalloproteinase inhibition lowers mortality and brain injury in experimental pneumococcal meningitis. Infect. Immun. 2014, 82, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Leib, S.L.; Clements, J.M.; Lindberg, R.L.; Heimgartner, C.; Loeffler, J.M.; Pfister, L.A.; Täuber, M.G.; Leppert, D. Inhibition of matrix metalloproteinases and tumour necrosis factor alpha converting enzyme as adjuvant therapy in pneumococcal meningitis. Brain 2001, 124 Pt 9, 1734–1742. [Google Scholar] [CrossRef]
- Liechti, F.D.; Bächtold, F.; Grandgirard, D.; Leppert, D.; Leib, S.L. The matrix metalloproteinase inhibitor RS-130830 attenuates brain injury in experimental pneumococcal meningitis. J. Neuroinflamm. 2015, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Barichello, T.; Generoso, J.S.; Michelon, C.M.; Simões, L.R.; Elias, S.G.; Vuolo, F.; Comim, C.M.; Dal-Pizzol, F.; Quevedo, J. Inhibition of matrix metalloproteinases-2 and -9 prevents cognitive impairment induced by pneumococcal meningitis in Wistar rats. Exp. Biol. Med. 2014, 239, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Meli, D.N.; Christen, S.; Leib, S.L.; Täuber, M.G. Current concepts in the pathogenesis of meningitis caused by Streptococcus pneumoniae. Curr. Opin. Infect. Dis. 2002, 15, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.S.; Garber, C.; Howard, N. Infectious immunity in the central nervous system and brain function. Nat. Immunol. 2017, 18, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Koedel, U.; Kastenbauer, S.; Pfister, H.W. Nitrogen and oxygen molecules in meningitis-associated labyrinthitis and hearing impairment. Infection 2008, 36, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Hogen, T.; Demel, C.; Giese, A.; Angele, B.; Pfister, H.W.; Koedel, U.; Klein, M. Adjunctive N-acetyl-L-cysteine in treatment of murine pneumococcal meningitis. Antimicrob. Agents Chemother. 2013, 57, 4825–4830. [Google Scholar] [CrossRef] [PubMed]
- Hofer, S.; Grandgirard, D.; Burri, D.; Fröhlich, T.K.; Leib, S.L. Bacterial meningitis impairs hippocampal neurogenesis. J. Neuropathol. Exp. Neurol. 2011, 70, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.A.; Vissel, B. The meteorology of cytokine storms, and the clinical usefulness of this knowledge. Semin. Immunopathol. 2017, 39, 505–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuster-Matanzo, A.; Llorens-Martín, M.; Hernández, F.; Avila, J. Role of neuroinflammation in adult neurogenesis and Alzheimer disease: Therapeutic approaches. Mediat. Inflamm. 2013, 2013, 260925. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.D.; Schoffelmeer, A.N.; De Vries, T.J.; Wardeh, G.; Dogterom, G.; Bol, J.G.; Binnekade, R.; Tilders, F.J.; et al. A single administration of interleukin-1 or amphetamine induces long-lasting increases in evoked noradrenaline release in the hypothalamus and sensitization of ACTH and corticosterone responses in rats. Eur. J. Neurosci. 2001, 13, 1923–1930. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, M.B.; Paik, K.D.; Caraway, J.D.; Schiml, P.A.; Deak, T. Proinflammatory activity and the sensitization of depressive-like behavior during maternal separation. Behav. Neurosci. 2011, 125, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.H.; Silver, R. Neuroimmune Signaling: Cytokines and the Central Nervous System. In Neuroscience in the 21st Century: From Basic to Clinical; Pfaff, D.W., Volkow, N.D., Eds.; Springer: New York, NY, USA, 2016; pp. 601–641. [Google Scholar]
- Barichello, T.; Generoso, J.S.; Simões, L.R.; Sharin, V.G.; Ceretta, R.A.; Dominguini, D.; Comim, C.M.; Vilela, M.C.; Teixeira, A.L.; Quevedo, J. Interleukin-1beta Receptor Antagonism Prevents Cognitive Impairment Following Experimental Bacterial Meningitis. Curr. Neurovasc. Res. 2015, 12, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.L. Epigenetic mechanisms in the development of behavior: Advances, challenges, and future promises of a new field. Dev. Psychopathol. 2013, 25, 1279–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Too, L.K. Acute Brain Pathology and Long-Term Neurological Sequelae in Experimental Pneumococcal Meningitis. PhD Thesis, University of Sydney, Sydney, Australia, 2014; pp. 232–237. [Google Scholar]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brouwer, M.C.; McIntyre, P.; Prasad, K.; van de Beek, D. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst. Rev. 2015, Cd004405. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Han, Q.; Sun, R.; Li, Z. Dexamethasone regulation of matrix metalloproteinase expression in experimental pneumococcal meningitis. Brain Res. 2008, 1207, 237–243. [Google Scholar] [CrossRef] [PubMed]



| Virulence Factor | Mode(s) of Action | Reference(s) | |
|---|---|---|---|
| Capsule | Thick polysaccharide capsule | Reduce neutrophil extracellular trapping | [43] |
| Reduce phagocytosis | [44] | ||
| Reduce complement deposition | [45] | ||
| Thin polysaccharide capsule | Expose pneumococcal surface protein binding sites | [43,44,45] | |
| Pili | Pneumococcal pilus-1 | Increase attachment to endothelial cells | [51] |
| Pilus adhesin RrgA | Facilitates BBB translocation through pIgR and PECAM-1 binding | [51] | |
| Choline binding proteins | Pneumococcal surface protein A (PspA) | Interferes with complement factor B | [53] |
| Inhibits human apo-lactoferrin activity | [56] | ||
| Choline-binding protein A (CbpA) | Inactivates C3b through complementary factor H binding | [54,55] | |
| Facilitates BBB translocation through plgR and PAF receptors on endothelial cells | [38,51,60] | ||
| Cell wall | Peptidoglycan | Activates host TLR, increasing inflammation | [66] |
| Teichoic Acid | Binds choline-binding proteins to pneumococcal cell wall | [36] | |
| Activates host TLR, increasing inflammation | [66] | ||
| Pneumococcal surface proteins | Neuraminidase A | Cleaves N-acetylneuraminic acid | [67] |
| Facilitates endothelial binding through LGLD | [52] | ||
| Secreted | Pneumococcal IgA protease | Cleaves host secretory IgA | [68] |
| Hyaluronidase | Degrades hyaluronan | [69] | |
| Pneumococcal phospholipase A2 (PLA2) | Increases inflammation | [63] | |
| Associated with upregulation of endothelial cell adhesins | [65] | ||
| Hydrogen peroxide | Kills competing microbes | [71] | |
| Pneumolysin | Cytolytic through ply pore formation to epithelial, endothelial and glial cells | [74,81,82,83,84] | |
| Stimulates complement pathways | [75] | ||
| Activates TLR and NLR inflammasome pathways | [76,77] | ||
| Activates NADPH oxidase | [78] | ||
| Activates ROS production in neutrophils | [78] | ||
| Disrupts ependymal cilia | [79,80] |
| Mediator | Associated Immune Consequence in PM | Reference(s) | |
|---|---|---|---|
| Chemokines | CCL2 | Monocyte, neutrophil and T-cell recruitment | [120] |
| CCL3 | |||
| CXCL8 | |||
| CXCL1 | Monocyte, neutrophil and T-cell recruitmentNatural Killer cell recruitment | ||
| CXCL3 | Natural Killer cell recruitment | ||
| CXCL12 | Activates endothelial cell integrins to induce leukocyte adhesion | [121,122] | |
| CCL11 | |||
| CCL21 | |||
| Cytokines | TNF | Induces neutrophil infiltrationIncreased BBB breakdown | [134] |
| IL-1β | Regulates inflammatory cytokines | [143] | |
| IL-6 | Increased BBB permeability | [137] | |
| IFN-γ | Activates macrophages and T-cellsRegulates inflammatory cytokinesIncreases BBB permeability | [110,143,144] | |
| CASP1 | Regulates IL-1β | [138] | |
| CASP3 | Increases hippocampal apoptosis | [156] | |
| RONS | H2O2 | Increases neuronal damage | [149] |
| Increases lipid peroxidation | [145] | ||
| Increases and activates MMPs | [150] | ||
| Increases brain oedema | [152,153] | ||
| eNOX | Protects against BBB damage | [147] | |
| NOS1 | Reduces leukocyte infiltration into the CNSProtects against BBB damage | [133] | |
| NOS2 | Increases serum nitriteIncreases leukocyte infiltration into the CNSIncreases BBB permeability | [132,158] | |
| MMPs | MMP2 | Increases BBB permeability | [167] |
| MMP9 | Increases BBB permeabilityIncreases neuronal apoptosis | [133,162,165] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yau, B.; Hunt, N.H.; Mitchell, A.J.; Too, L.K. Blood‒Brain Barrier Pathology and CNS Outcomes in Streptococcus pneumoniae Meningitis. Int. J. Mol. Sci. 2018, 19, 3555. https://doi.org/10.3390/ijms19113555
Yau B, Hunt NH, Mitchell AJ, Too LK. Blood‒Brain Barrier Pathology and CNS Outcomes in Streptococcus pneumoniae Meningitis. International Journal of Molecular Sciences. 2018; 19(11):3555. https://doi.org/10.3390/ijms19113555
Chicago/Turabian StyleYau, Belinda, Nicholas H. Hunt, Andrew J. Mitchell, and Lay Khoon Too. 2018. "Blood‒Brain Barrier Pathology and CNS Outcomes in Streptococcus pneumoniae Meningitis" International Journal of Molecular Sciences 19, no. 11: 3555. https://doi.org/10.3390/ijms19113555
APA StyleYau, B., Hunt, N. H., Mitchell, A. J., & Too, L. K. (2018). Blood‒Brain Barrier Pathology and CNS Outcomes in Streptococcus pneumoniae Meningitis. International Journal of Molecular Sciences, 19(11), 3555. https://doi.org/10.3390/ijms19113555