Ethinylestradiol and Levonorgestrel as Active Agents in Normal Skin, and Pathological Conditions Induced by UVB Exposure: In Vitro and In Ovo Assessments
Abstract
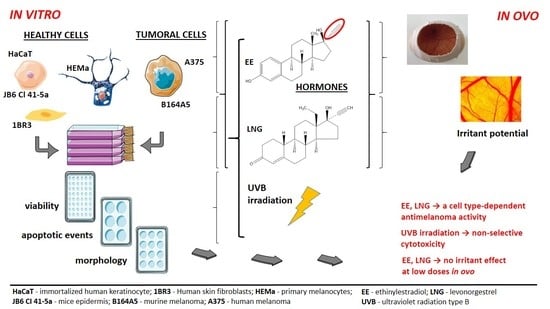
:1. Introduction
2. Results
2.1. Ethinylestradiol and Levonorgestrel ± UVB Irradiation Induced Differential Effects on Healthy Cell and Tumor Cell Viability
2.2. Ethinylestradiol and Levonorgestrel ± UVB Irradiation Triggered Apoptosis in A375 and B164A5 Melanoma Cells
2.3. Ethinylestradiol (EE) and Levonorgestrel (LNG) ± UVB Irradiation Determined Changes in Cells Morphology
2.4. The impact of Ethinylestradiol (EE) and Levonorgestrel (LNG) on Healthy and Tumor Cells Migration and Proliferation
2.5. Irritant Potential Assessment of Ethinylestradiol and Levonorgestrel by the Means of a HET-CAM Assay
3. Discussion
4. Materials and Methods
4.1. Reagents and Cell Lines
4.2. Cell Culture
4.3. UVB Irradiation Protocol
4.4. Cell Viability, Migration and Proliferation Assays
4.5. Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM) Assay
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| A375 | Human melanoma cells |
| B164A5 | Murine melanoma cells |
| BCC | Basal cell carcinoma |
| 1BR3 | Human skin fibroblasts |
| DMSO | Dimethyl sulfoxide |
| EE | Ethinylestradiol |
| ER | Estrogen receptor |
| ET-1 | Endothelin-1 |
| HaCaT | Human immortalized keratinocytes |
| HEMa | Human primary epidermal melanocytes |
| HET-CAM | Hen’s Egg Test—chick chorioallantoic membrane assay |
| ICCVAM | Interagency Coordinating Committee on the Validation of Alternative Methods |
| IS | Irritation score |
| JB6Cl415a | Newborn mice epidermis |
| LNG | Levonorgestrel |
| MSH | Melanocyte-stimulating hormone |
| PBS | Phosphate saline buffer |
| SCC | Squamous cell carcinoma |
| SCF | Stem cell factor |
| SDS | Sodium dodecyl sulfate |
| SS | Severity score |
| UVB | Ultraviolet B radiation |
| UVA | Ultraviolet A radiation |
References
- Burkman, R.; Schlesselman, J.J.; Zieman, M. Safety concerns and health benefits associated with oral contraception. Am. J. Obstet. Gynecol. 2004, 190 (Suppl. 4), S5–S22. [Google Scholar] [CrossRef] [PubMed]
- Stanczyk, F.Z.; Archer, D.F.; Bhavnani, B.R. Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: Pharmacokinetics, pharmacodynamics and risk assessment. Contraception 2013, 87, 706–727. [Google Scholar] [CrossRef] [PubMed]
- Leslie, K.K.; Espey, E. Oral contraceptives and skin cancer: Is there a link? Am. J. Clin. Dermatol. 2005, 6, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Dhont, M. History of oral contraception. Eur. J. Contracept. Reprod. Health Care 2010, 15 (Suppl. 2), S12–S18. [Google Scholar] [CrossRef] [PubMed]
- Lata, K.; Mukherjee, T.K. Knockdown of receptor for advanced glycation end products attenuate 17α-ethinyl-estradiol dependent proliferation and survival of MCF-7 breast cancer cells. Biochim. Biophys. Acta 2014, 1840, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Shin, S.J.; Yoo, Y.; Kim, N.H.; Kim, D.S.; Zhang, D.; Park, J.A.; Yi, H.; Kim, J.S.; Shin, H.C. Oral toxicity of isotretinoin, misoprostol, methotrexate, mifepristone and levonorgestrel as pregnancy category X medications in female mice. Exp. Ther. Med. 2015, 9, 853–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iversen, L.; Sivasubramaniam, S.; Lee, A.J.; Fielding, S.; Hannaford, P.C. Lifetime cancer risk and combined oral contraceptives: The Royal College of General Practitioners’ Oral Contraception Study. Am. J. Obstet. Gynecol. 2017, 216, 580.e1–580.e9. [Google Scholar] [CrossRef] [PubMed]
- Janik, M.E.; Bełkot, K.; Przybyło, M. Is oestrogen an important player in melanoma progression? Contemp. Oncol. (Pozn) 2014, 18, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Marzagalli, M.; Montagnani Marelli, M.; Casati, L.; Fontana, F.; Moretti, R.M.; Limonta, P. Estrogen Receptor β in Melanoma: From Molecular Insights to Potential Clinical Utility. Front. Endocrinol. (Lausanne) 2016, 7, 140. [Google Scholar] [CrossRef] [PubMed]
- Dika, E.; Fanti, P.A.; Vaccari, S.; Capizzi, E.; Degiovanni, A.; Gobbi, A.; Piraccini, B.M.; Ribero, S.; Baraldi, C.; Ravaioli, G.M.; et al. Oestrogen and progesterone receptors in melanoma and nevi: An immunohistochemical study. Eur. J. Dermatol. 2017, 27, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Kuklinski, L.F.; Zens, M.S.; Perry, A.E.; Gossai, A.; Nelson, H.H.; Karagas, M.R. Sex hormones and the risk of keratinocyte cancers among women in the United States: A population-based case-control study. Int. J. Cancer 2016, 139, 300–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coricovac, D.; Dehelean, C.; Moaca, E.A.; Pinzaru, I.; Bratu, T.; Navolan, D.; Boruga, O. Cutaneous Melanoma—A Long Road from Experimental Models to Clinical Outcome: A Review. Int. J. Mol. Sci. 2018, 19, 1566. [Google Scholar] [CrossRef] [PubMed]
- Roh, M.R.; Eliades, P.; Gupta, S.; Grant-Kels, J.M.; Tsao, H. Cutaneous melanoma in women. Int. J. Women’s Dermatol. 2017, 3 (Suppl. 1), S11–S15. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.L.; Fernandez, A.A.; Garcia, R.; Paniker, L.; Lin, K.; Hanninen, A.; Zigelsky, K.; May, M.; Nuttall, M.; Lo, H.H.; et al. Acute exposure to ultraviolet-B radiation modulates sex steroid hormones and receptor expression in the skin and may contribute to the sex bias of melanoma in a fish model. Pigment Cell Melanoma Res. 2014, 27, 408–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tchernev, G.; Dzhelyatova, G.A.; Wollina, U.; Lozev, I.; Lotti, T. Medium Sized Congenital Melanocytic Nevus with Suspected Progression to Melanoma during Pregnancy: What’s the Best for the Patient? J. Med. Sci. 2018, 6, 143–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smalley, K.S. Why do women with melanoma do better than men? eLife 2018, 7, E33511. [Google Scholar] [CrossRef] [PubMed]
- Tamega Ade, A.; Miot, H.A.; Moço, N.P.; Silva, M.G.; Marques, M.E.; Miot, L.D. Gene and protein expression of oestrogen-β and progesterone receptors in facial melasma and adjacent healthy skin in women. Int. J. Cosmet. Sci. 2015, 37, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.R. Melasma treatment: A novel approach using a topical agent that contains an anti-estrogen and a vascular endothelial growth factor inhibitor. Med. Hypotheses 2017, 101, 1–5. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, V.; Gori, A.; Grazzini, M.; Rossari, S.; Scarfì, F.; Corciova, S.; Verdelli, A.; Lotti, T.; Massi, D. Estrogens, estrogen receptors and melanoma. Expert Rev. Anticancer Ther. 2011, 11, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, G.; Ren, L. Localization of sex steroid receptors in human skin. Histol. Histopathol. 2004, 19, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Botteri, E.; Støer, N.C.; Sakshaug, S.; Graff-Iversen, S.; Vangen, S.; Hofvind, S.; Ursin, G.; Weiderpass, E. Menopausal hormone therapy and risk of melanoma: Do estrogens and progestins have a different role? Int. J. Cancer 2017, 141, 1763–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natale, C.A.; Duperret, E.K.; Zhang, J.; Sadeghi, R.; Dahal, A.; O’Brien, K.T.; Cookson, R.; Winkler, J.D.; Ridky, T.W. Sex steroids regulate skin pigmentation through nonclassical membrane-bound receptors. eLife 2016, 5, E15104. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, M.; Tropea, S.; Rossi, C.R.; Alaibac, M. Melanoma: Epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo 2014, 28, 1005–1011. [Google Scholar] [PubMed]
- Leslie, K.S.; Lodge, E.; Garioch, J.J. A comparison of narrowband (TL-01) UVB-induced erythemal response at different body sites. Clin. Exp. Dermatol. 2005, 30, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M. DNA hypomethylation in cancer cells. Epigenomics 2009, 1, 239–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richarz, N.A.; Aguilera, J.; Castillo, G.; Fuente, M.J.; Ferrándiz, C.; Carrascosa, J.M. Phototoxic reaction to a combined oral contraceptive (levonorgestrel/ethinylestradiol). Photochem. Photobiol. Sci. 2017, 16, 1381–1383. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Shin, C.Y.; Kim, Y.K.; Lee, S.R.; Chung, J.H. Endogenous estrogen exacerbates UV-induced inflammation and photoaging in mice. J. Investig. Dermatol. 2014, 134, 2290–2293. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Chung, J.H. Long-Term Estrogen Effects on Sun-Exposed Human Skin in M.A. In Textbook of Aging Skin; Farage, M., Miller, K., Maibach, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Röck, K.; Joosse, S.A.; Müller, J.; Heinisch, N.; Fuchs, N.; Meusch, M.; Zipper, P.; Reifenberger, J.; Pantel, K.; Fischer, J.W. Chronic UVB-irradiation actuates perpetuated dermal matrix remodeling in female mice: Protective role of estrogen. Sci. Rep. 2016, 6, 30482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Kim, T.K.; Brożyna, A.A.; Janjetovic, Z.; Brooks, D.L.; Schwab, L.P.; Skobowiat, C.; Jóźwicki, W.; Seagroves, T.N. The role of melanogenesis in regulation of melanoma behavior: Melanogenesis leads to stimulation of HIF-1α expression and HIF-dependent attendant pathways. Arch. Biochem. Biophys. 2014, 563, 79–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slominski, R.M.; Zmijewski, M.A.; Slominski, A.T. The role of melanin pigment in melanoma. Exp. Dermatol. 2015, 24, 258–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brożyna, A.A.; Jóźwicki, W.; Roszkowski, K.; Filipiak, J.; Slominski, A.T. Melanin content in melanoma metastases affects the outcome of radiotherapy. Oncotarget 2016, 7, 17844–17853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brożyna, A.A.; Jóźwicki, W.; Carlson, J.A.; Slominski, A.T. Melanogenesis affects overall and disease-free survival in patients with stage III and IV melanoma. Hum. Pathol. 2013, 44, 2071–2074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM 2010). Available online: https://www.niehs.nih.gov/health/materials/interagency_coordinating_committee_on_the_validation_of_alternative_methods_508.pdf (accessed on 1 August 2018).
- Gallagher, R.P.; Elwood, J.M.; Hill, G.B.; Coldman, A.J.; Threlfall, W.J.; Spinelli, J.J. Reproductive factors, oral contraceptives and risk of malignant melanoma: Western Canada Melanoma Study. Br. J. Cancer 1985, 52, 901–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holman, C.D.; Armstrong, B.K.; Heenan, P.J. Cutaneous malignant melanoma in women: Exogenous sex hormones and reproductive factors. Br. J. Cancer 1984, 50, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Fine, J.A.; Barnhill, R.L.; Berwick, M. Hormonal and reproductive influences and risk of melanoma in women. Int. J. Epidemiol. 1998, 27, 751–757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koomen, E.R.; Joosse, A.; Herings, R.M.; Casparie, M.K.; Guchelaar, H.J.; Nijsten, T. Estrogens, oral contraceptives and hormonal replacement therapy increase the incidence of cutaneous melanoma: A population-based case-control study. Ann. Oncol. 2009, 20, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Moroni, G.; Gaziano, R.; Bue, C.; Agostini, M.; Perno, C.F.; Sinibaldi-Vallebona, P.; Pica, F. Progesterone and Melanoma Cells: An Old Story Suspended between Life and Death. J. Steroids Horm. Sci. 2015, S13, 158. [Google Scholar] [CrossRef]
- Karagas, M.R.; Stukel, T.A.; Dykes, J.; Miglionico, J.; Greene, M.A.; Carey, M.; Armstrong, B.; Elwood, J.M.; Gallagher, R.P.; Green, A.; et al. A pooled analysis of 10 case-control studies of melanoma and oral contraceptive use. Br. J. Cancer 2002, 86, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.; Verzi, A.E.; Bhatt, K.; Orrell, K.; Hagstorm, E.; Flood, K.; Schlosser, B.; Nardone, B.; West, D.P. Melanoma and chronic exposure to contraceptives containing microdoses of ethinylestradiol in young women: A retrospective study from the Research on Adverse Drug Events and Reports (RADAR) project comprising a large Midwestern U.S. patient population. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e87–e88. [Google Scholar] [CrossRef] [PubMed]
- Bhupathiraju, S.N.; Grodstein, F.; Stampfer, M.J.; Willett, W.C.; Hu, F.B.; Manson, J.E. Exogenous Hormone Use: Oral Contraceptives, Postmenopausal Hormone Therapy, and Health Outcomes in the Nurses’ Health Study. Am. J. Public Health 2016, 106, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Mordoh, J.; Tapia, I.J.; Barrio, M.M. A word of caution: Do not wake sleeping dogs; micrometastases of melanoma suddenly grew after progesterone treatment. BMC Cancer 2013, 13, 132. [Google Scholar] [CrossRef] [PubMed]
- Caraffa, A.; Spinas, E.; Kritas, S.K.; Lessiani, G.; Ronconi, G.; Saggini, A.; Antinolfi, P.; Pizzicannella, J.; Toniato, E.; Theoharides, T.C.; et al. Endocrinology of the skin: Intradermal neuroimmune network, a new frontier. J. Biol. Regul. Homeost. Agents 2016, 30, 339–343. [Google Scholar] [PubMed]
- Muizzuddin, N.; Marenus, K.D.; Schnittger, S.F.; Sullivan, M.; Maes, D.H. Effect of systemic hormonal cyclicity on skin. J. Cosmet. Sci. 2005, 56, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Carnesecchi, J.; Malbouyres, M.; de Mets, R.; Balland, M.; Beauchef, G.; Vié, K.; Chamot, C.; Lionnet, C.; Ruggiero, F.; Vanacker, J.M. Estrogens induce rapid cytoskeleton re-organization in human dermal fibroblasts via the non-classical receptor GPR30. PLoS ONE 2015, 10, e0120672. [Google Scholar] [CrossRef] [PubMed]
- Natale, C.A.; Li, J.; Zhang, J.; Dahal, A.; Dentchev, T.; Stanger, B.Z.; Ridky, T.W. Activation of G protein-coupled estrogen receptor signaling inhibits melanoma and improves response to immune checkpoint blockade. eLife 2018, 7, E31770. [Google Scholar] [CrossRef] [PubMed]
- Ramaraj, P.; Cox, J.L. In vitro effect of progesterone on human melanoma (BLM) cell growth. Int. J. Clin. Exp. Med. 2014, 7, 3941–3953. [Google Scholar] [PubMed]
- Fernandez, T.L.; Van Lonkhuyzen, D.R.; Dawson, R.A.; Kimlin, M.G.; Upton, Z. In vitro investigations on the effect of dermal fibroblasts on keratinocyte responses to ultraviolet B radiation. Photochem. Photobiol. 2014, 90, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Duval, C.; Cohen, C.; Chagnoleau, C.; Flouret, V.; Bourreau, E.; Bernerd, F. Key Regulatory Role of Dermal Fibroblasts in Pigmentation as Demonstrated Using a Reconstructed Skin Model: Impact of Photo-Aging. PLoS ONE 2014, 9, e114182. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, C.; Nägele, U.; Schramm, G.; Berking, C. Inhibitory effects of progestogens on the estrogen stimulation of melanocytes in vitro. Contraception 2009, 80, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Kanda, N.; Watanabe, S. 17beta-estradiol stimulates the growth of human keratinocytes by inducing cyclin D2 expression. J. Investig. Dermatol. 2004, 123, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Poletini, M.O.; de Assis, L.V.; Moraes, M.N.; Castrucci, A.M. Estradiol differently affects melanin synthesis of malignant and normal melanocytes: A relationship with clock and clock-controlled genes. Mol. Cell Biochem. 2016, 421, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Izykowska, I.; Cegielski, M.; Gebarowska, E.; Podhorska-Okolow, M.; Piotrowska, A.; Zabel, M.; Dziegiel, P. Effect of melatonin on human keratinocytes and fibroblasts subjected to UVA and UVB radiation In vitro. In Vivo 2009, 23, 739–745. [Google Scholar] [PubMed]
- Mortensen, L.J.; Ravichandran, S.; DeLouise, L.A. The impact of UVB exposure and differentiation state of primary keratinocytes on their interaction with quantum dots. Nanotoxicology 2013, 7, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.T.; Cha, H.J.; Lee, K.S.; Lee, K.K.; Hong, J.T.; Ahn, K.J.; An, I.S.; An, S.; Bae, S. Arctiin induces an UVB protective effect in human dermal fibroblast cells through microRNA expression changes. Int. J. Mol. Med. 2014, 33, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, J.; Bhatt, P. Ultraviolet-B Protective Effect of Flavonoids from Eugenia caryophylata on Human Dermal Fibroblast Cells. Pharmacogn. Mag. 2015, 11 (Suppl. 3), S397–S406. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Kim, A.; Nakatani, M.; Shen, Y.; Liu, L. Distinctive molecular responses to ultraviolet radiation between keratinocytes and melanocytes. Exp. Dermatol. 2016, 25, 708–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, D.; Lee, S.; Huang, Y.H.; Lim, H.W.; Lee, Y.; Jang, K.; Cho, Y.; Park, S.J.; Kim, D.D.; Lim, C.J. Protective properties of geniposide against UV-B-induced photooxidative stress in human dermal fibroblasts. Pharm. Biol. 2018, 56, 176–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarti, M.S.; Visconti, M.A.; Castrucci, A.M. Biological activity and binding of estradiol to SK-Mel 23 human melanoma cells. Braz. J. Med. Biol. Res. 2004, 37, 901–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serafim, V.; Shah, A.; Puiu, M.; Andreescu, N.; Coricovac, D.; Nosyrev, A.; Spandidos, D.A.; Tsatsakis, A.M.; Dehelean, C.; Pinzaru, I. Classification of cancer cell lines using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and statistical analysis. Int. J. Mol. Med. 2017, 40, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.M.; Lee, J.E.; Kim, S.Y.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Kim, D.S. Enhanced effects of citrate on UVB-induced apoptosis of B16 melanoma cells. Pharmazie 2009, 64, 829–833. [Google Scholar] [PubMed]
- Wang, W.Q.; Wu, J.F.; Xiao, X.Q.; Xiao, Q.; Wang, J.; Zuo, F.G. Narrow-band UVB radiation promotes dendrite formation by activating Rac1 in B16 melanoma cells. Mol. Clin. Oncol. 2013, 1, 858–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, T.D.; Steck, W.F. A modified HET-CAM assay approach to the assessment of anti-irritant properties of plant extracts. Food Chem. Toxicol. 2000, 38, 867–872. [Google Scholar] [CrossRef]
- Rocha-Filho, P.; Ferrari, M.; Maruno, M.; Souza, O.; Gumiero, V. In Vitro and In Vivo Evaluation of Nanoemulsion Containing Vegetable Extracts. Cosmetics 2017, 4, 32. [Google Scholar] [CrossRef]
- Palmeira-de-Oliveira, R.; Monteiro Machado, R.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, A. Testing vaginal irritation with the hen’s egg test-chorioallantoic membrane assay. ALTEX 2018. [Google Scholar] [CrossRef] [PubMed]
- Wieder, D.R.; Pattimakiel, L. Examining the efficacy, safety, and patient acceptability of the combined contraceptive vaginal ring (NuvaRing). Int. J. Women’s Health 2010, 2, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.; Mobedi, H.; Mosaffa, N.; Dolatian, M.; Ramezani Tehrani, F. Hormone Therapy for Relieving Postmenopausal Vasomotor Symptoms: A Systematic Review. Arch. Iran Med. 2016, 19, 141–146. [Google Scholar] [PubMed]
- Torgrimson, B.N.; Meendering, J.R.; Kaplan, P.F.; Minson, C.T. Endothelial function across an oral contraceptive cycle in women using levonorgestrel and ethinyl estradiol. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2874–H2880. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.E.; Wang, Y.T.; Lu, C.Y.; Fang, A.H.; Wu, C.S. The effect of interaction of heat and UVB on human keratinocyte: Novel insights on UVB-induced carcinogenesis of the skin. J. Dermatol. Sci. 2017, 88, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Coricovac, D.E.; Moacă, E.A.; Pinzaru, I.; Cîtu, C.; Soica, C.; Mihali, C.V.; Păcurariu, C.; Tutelyan, V.A.; Tsatsakis, A.; Dehelean, C.A. Biocompatible Colloidal Suspensions Based on Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Toxicological Profile. Front. Pharmacol. 2017, 8, 154. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Weigert, A.; Tausendschön, A.; Mora, J.; Oren, B.; Sola, A.; Hotter, G.; Muta, T.; Brüne, B. Interleukin-10-induced neutrophil gelatinase-associated lipocalin production in macrophages with consequences for tumor growth. Mol. Cell Biol. 2012, 32, 3938–3948. [Google Scholar] [CrossRef] [PubMed]
- Felice, F.; Zambito, Y.; Belardinelli, E.; Fabiano, A.; Santoni, T.; Di Stefano, R. Effect of different chitosan derivatives on in vitro scratch wound assay: A comparative study. Int. J. Biol. Macromol. 2015, 76, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Luepke, N.P. Hen’s egg chorioallantoic membrane test for irritation potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef]
- Scheel, J.; Kleber, M.; Kreutz, J.; Lehringer, E.; Mehling, A.; Reisinger, K.; Steiling, W. Eye irritation potential: Usefulness of the HET-CAM under the Globally Harmonized System of classification and labeling of chemicals (GHS). Regul. Toxicol. Pharmacol. 2011, 59, 471–492. [Google Scholar] [CrossRef] [PubMed]
- Ardelean, S.; Feflea, S.; Ionescu, D.; Năstase, V.; Dehelean, C.A. Toxicologic screening of some surfactants using modern in vivo bioassays. Rev. Med. Chir. Soc. Med. Nat. Iasi 2011, 115, 251–258. [Google Scholar] [PubMed]














| Test Compound and Controls | Irritation Score (Mean) | Irritation Severity (Mean) | Classification of the Effect |
|---|---|---|---|
| PBS Negative control | 0 ± 0 | 0 ± 0 | Non-irritant |
| SDS Positive control | 15.07 ± 1.08 | 2.67 ± 0.58 | Strong irritant |
| DMSO 1% Solvent Control | 0 ± 0 | 0 ± 0 | Non-irritant |
| EE 1 µM | 0 ± 0 | 0 ± 0 | Non-irritant |
| EE 10 µM | 2.79 ± 0.55 | 1.33 ± 0.58 | Weak irritant |
| LNG 1 µM | 0 ± 0 | 0 ± 0 | Non-irritant |
| LNG 10 µM | 0 ± 0 | 0 ± 0 | Non-irritant |
| EE + LNG 1 µM | 0.63 ± 0.3 | 0.83 ± 0.29 | Non-irritant |
| EE + LNG 10 µM | 1.23 ± 0.3 | 1 ± 0 | Weak irritant |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coricovac, D.; Farcas, C.; Nica, C.; Pinzaru, I.; Simu, S.; Stoian, D.; Soica, C.; Proks, M.; Avram, S.; Navolan, D.; et al. Ethinylestradiol and Levonorgestrel as Active Agents in Normal Skin, and Pathological Conditions Induced by UVB Exposure: In Vitro and In Ovo Assessments. Int. J. Mol. Sci. 2018, 19, 3600. https://doi.org/10.3390/ijms19113600
Coricovac D, Farcas C, Nica C, Pinzaru I, Simu S, Stoian D, Soica C, Proks M, Avram S, Navolan D, et al. Ethinylestradiol and Levonorgestrel as Active Agents in Normal Skin, and Pathological Conditions Induced by UVB Exposure: In Vitro and In Ovo Assessments. International Journal of Molecular Sciences. 2018; 19(11):3600. https://doi.org/10.3390/ijms19113600
Chicago/Turabian StyleCoricovac, Dorina, Claudia Farcas, Cristian Nica, Iulia Pinzaru, Sebastian Simu, Dana Stoian, Codruta Soica, Maria Proks, Stefana Avram, Dan Navolan, and et al. 2018. "Ethinylestradiol and Levonorgestrel as Active Agents in Normal Skin, and Pathological Conditions Induced by UVB Exposure: In Vitro and In Ovo Assessments" International Journal of Molecular Sciences 19, no. 11: 3600. https://doi.org/10.3390/ijms19113600
APA StyleCoricovac, D., Farcas, C., Nica, C., Pinzaru, I., Simu, S., Stoian, D., Soica, C., Proks, M., Avram, S., Navolan, D., Dumitru, C., Popovici, R. A., & Dehelean, C. A. (2018). Ethinylestradiol and Levonorgestrel as Active Agents in Normal Skin, and Pathological Conditions Induced by UVB Exposure: In Vitro and In Ovo Assessments. International Journal of Molecular Sciences, 19(11), 3600. https://doi.org/10.3390/ijms19113600