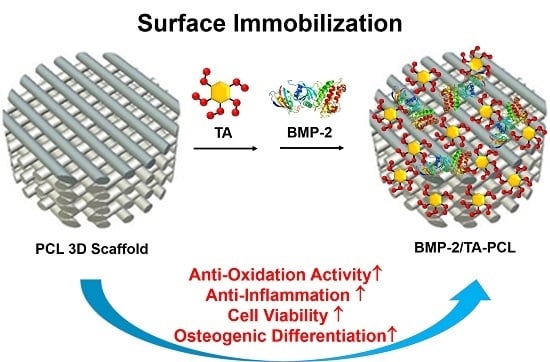
Design of a 3D BMP-2-Delivering Tannylated PCL Scaffold and Its Anti-Oxidant, Anti-Inflammatory, and Osteogenic Effects In Vitro
Abstract
:1. Introduction
2. Results
2.1. Preparation and Characterizations of PCL and the Functionalized PCL Scaffolds with TA and/or BMP-2
2.2. In Vitro Release of BMP-2 from Scaffolds
2.3. In Vitro Anti-Oxidant Study
2.4. ROS Scavenging Effects in Cells
2.5. Protection of Cell Viabilities against the ROS Condition
2.6. Anti-Inflammatory Effects of the Scaffolds on Lipopolysaccharide-Stimulated MC3T3-E1 Cells
2.7. Alkaline Phosphatase Activity
2.8. Calcium Deposition
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Fabrication of PCL Scaffolds
4.3. Fabrication of BMP-2/TA/PCL Scaffolds
4.4. Characterization of PCL and Modified PCL Scaffolds
4.5. SEM Observation of Cell Infiltration into the Scaffolds
4.6. In Vitro BMP-2 Release Study
4.7. Anti-Oxidant Studies
4.7.1. Anti-Oxidant Activity Assay
4.7.2. Measurement of ROS in the Cell Level
4.7.3. Protection of Cell Viabilities against the ROS Environment
4.8. Anti-Inflammatory Effects
4.9. ALP Activity and Calcium Deposition
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Seitz, H.; Rieder, W.; Irsen, S.; Leukers, B.; Tille, C. Three-dimensional printing of porous ceramic scaffolds for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 74, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Yun, Y.P.; Park, Y.E.; Lee, S.H.; Yong, W.; Kundu, J.; Jung, J.W.; Shim, J.H.; Cho, D.W.; Kim, S.E.; et al. In vitro and in vivo evaluation of bone formation using solid freeform fabrication-based bone morphogenic protein-2 releasing PCL/PLGA scaffolds. Biomed. Mater. 2014, 9, 025008. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.S.; Kim, S.E.; Yun, Y.P.; Jeon, D.I.; Kim, H.J.; Park, K.; Song, H.R. Surface immobilization of biphasic calcium phosphate nanoparticles on 3D printed poly(caprolactone) scaffolds enhances osteogenesis and bone tissue regeneration. J. Ind. Eng. Chem. 2017, 55, 101–109. [Google Scholar] [CrossRef]
- Peltola, S.M.; Melchels, F.P.; Grijpma, D.W.; Kellomaki, M. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 2008, 40, 268–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wust, S.; Muller, R.; Hofmann, S. Controlled positioning of cells in biomaterials-approaches towards 3D tissue printing. J. Funct. Biomater. 2011, 2, 119–154. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Han, L.; Martin-Millan, M.; O’Brien, C.A.; Manolagas, S.C. Oxidative stress antagonizes wnt signaling in osteoblast precursors by diverting beta-catenin from T cell factor- to forkhead box o-mediated transcription. J. Biol. Chem. 2007, 282, 27298–27305. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Ambrogini, E.; Han, L.; Manolagas, S.C.; Jilka, R.L. Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic wnt signaling in the skeleton. J. Biol. Chem. 2009, 284, 27438–27448. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Martin-Millan, M.; Ambrogini, E.; Bradsher, R., 3rd; Han, L.; Chen, X.D.; Roberson, P.K.; Weinstein, R.S.; O’Brien, C.A.; Jilka, R.L.; et al. Estrogens attenuate oxidative stress and the differentiation and apoptosis of osteoblasts by DNA-binding-independent actions of the eralpha. J. Bone Miner. Res. 2010, 25, 769–781. [Google Scholar] [PubMed]
- Bai, X.C.; Lu, D.; Bai, J.; Zheng, H.; Ke, Z.Y.; Li, X.M.; Luo, S.Q. Oxidative stress inhibits osteoblastic differentiation of bone cells by erk and nf-kappab. Biochem. Biophys. Res. Commun. 2004, 314, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Lean, J.M.; Davies, J.T.; Fuller, K.; Jagger, C.J.; Kirstein, B.; Partington, G.A.; Urry, Z.L.; Chambers, T.J. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J. Clin. Invest. 2003, 112, 915–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef] [PubMed]
- Kolambkar, Y.M.; Dupont, K.M.; Boerckel, J.D.; Huebsch, N.; Mooney, D.J.; Hutmacher, D.W.; Guldberg, R.E. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials 2011, 32, 65–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schofer, M.D.; Roessler, P.P.; Schaefer, J.; Theisen, C.; Schlimme, S.; Heverhagen, J.T.; Voelker, M.; Dersch, R.; Agarwal, S.; Fuchs-Winkelmann, S.; et al. Electrospun plla nanofiber scaffolds and their use in combination with bmp-2 for reconstruction of bone defects. PLoS ONE 2011, 6, e25462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.E.; Yun, Y.P.; Han, Y.K.; Lee, D.W.; Ohe, J.Y.; Lee, B.S.; Song, H.R.; Park, K.; Choi, B.J. Osteogenesis induction of periodontal ligament cells onto bone morphogenic protein-2 immobilized PCL fibers. Carbohydr. Polym. 2014, 99, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Yun, Y.P.; Shim, K.S.; Kim, H.J.; Park, K.; Song, H.R. 3D printed alendronate-releasing poly(caprolactone) porous scaffolds enhance osteogenic differentiation and bone formation in rat tibial defects. Biomed. Mater. 2016, 11, 055005. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine surface chemistry: A decade of discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Abouelmagd, S.A.; Meng, F.; Kim, B.K.; Hyun, H.; Yeo, Y. Tannic acid-mediated surface functionalization of polymeric nanoparticles. ACS Biomater. Sci. Eng. 2016, 2, 2294–2303. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Yun, Y.P.; Song, H.R.; Chun, H.J.; Yang, D.H.; Park, K.; Kim, S.E. The effect of titanium with heparin/bmp-2 complex for improving osteoblast activity. Carbohydr. Polym. 2013, 98, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Yun, Y.P.; Shim, K.S.; Park, K.; Choi, S.W.; Shin, D.H.; Suh, D.H. Fabrication of a bmp-2-immobilized porous microsphere modified by heparin for bone tissue engineering. Colloids. Surf. B Biointerfaces 2015, 134, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Yeon, J.; Song, I.T.; Kang, S.M.; Lee, H.; Lee, H. Pyrogallol 2-aminoethane: A plant flavonoid-inspired molecule for material-independent surface chemistry. Adv. Mater. Interfaces 2014, 1, 1400113. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Aktas, N.; Silan, C. Inherently antioxidant and antimicrobial tannic acid release from poly(tannic acid) nanoparticles with controllable degradability. Colloids. Surf. B Biointerfaces 2016, 142, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Lee, H.-A.; Lee, M.; Shin, Y.; Song, J.-J.; Kang, S.-W.; Nam, D.-H.; Jeon, E.J.; Cho, M.; Do, M.; et al. Targeting protein and peptide therapeutics to the heart via tannic acid modification. Nat. Biomed. Eng. 2018, 2, 304–317. [Google Scholar] [CrossRef]
- Turgut Cosan, D.; Saydam, F.; Ozbayer, C.; Doganer, F.; Soyocak, A.; Gunes, H.V.; Degirmenci, I.; Kurt, H.; Ustuner, M.C.; Bal, C. Impact of tannic acid on blood pressure, oxidative stress and urinary parameters in l-nna-induced hypertensive rats. Cytotechnology 2015, 67, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Labieniec, M.; Gabryelak, T. Oxidatively modified proteins and DNA in digestive gland cells of the fresh-water mussel unio tumidus in the presence of tannic acid and its derivatives. Mutat. Res. 2006, 603, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.G., Jr.; Dalvi, L.T.; Silva, J.M., Jr.; Lopes, G.K.; Alonso, A.; Hermes-Lima, M. The antioxidant effect of tannic acid on the in vitro copper-mediated formation of free radicals. Arch. Biochem. Biophys. 2005, 437, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.K.; Schulman, H.M.; Hermes-Lima, M. Polyphenol tannic acid inhibits hydroxyl radical formation from fenton reaction by complexing ferrous ions. Biochim. Biophys. Acta 1999, 1472, 142–152. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and anti-inflammatory ph-responsive tannic acid-carboxylated agarose composite hydrogels for wound healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.M.; Hwang, K.; Winn, S.R.; Hollinger, J.O. Bone morphogenetic proteins: An update on basic biology and clinical relevance. J. Orthop. Res. 1999, 17, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Ebara, S.; Nakayama, K. Mechanism for the action of bone morphogenetic proteins and regulation of their activity. Spine (Phila Pa 1976) 2002, 15, S10–S15. [Google Scholar] [CrossRef]
- Reddi, A.H. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat. Biotechnol. 1998, 16, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Yamamoto, M.; Tabata, Y. Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and beta-tricalcium phosphate. Biomaterials 2005, 26, 4856–4865. [Google Scholar] [CrossRef] [PubMed]
- Geissler, S.; Barrantes, A.; Tengvall, P.; Messersmith, P.B.; Tiainen, H. Deposition kinetics of bioinspired phenolic coatings on titanium surfaces. Langmuir 2016, 32, 8050–8060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isenburg, J.C.; Karamchandani, N.V.; Simionescu, D.T.; Vyavahare, N.R. Structural requirements for stabilization of vascular elastin by polyphenolic tannins. Biomaterials 2006, 27, 3645–3651. [Google Scholar] [CrossRef] [PubMed]
- Ozecelik, B.; Lee, J.H.; Min, D.B. Effects of light, oxygen, and ph on the absorbance of 2,2-diphenyl-1-picrylhydrazyl. J. Food. Sci. 2006, 68, 487–490. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Goli, S.A.H.; Fathi, M. Fabrication and characterization of electrospun gelatin nanofibers crosslinked with oxidized phenolic compounds. Int. J. Biol. Macromol. 2017, 103, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Nohl, H. Involvement of free radicals in ageing: A consequence or cause of senescence. Br. Med. Bull. 1993, 49, 653–667. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Ranjbar, A.; Shadnia, S.; Nikfar, S.; Rezaie, A. Pesticides and oxidative stress: A review. Med. Sci. Monit. 2004, 10, RA141–RA147. [Google Scholar] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, M.; Kato, J.; Moss, J. Adp-ribosyl-acceptor hydrolase 3 regulates poly (adp-ribose) degradation and cell death during oxidative stress. Proc. Natl. Acad. Sci. USA 2013, 110, 18964–18969. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Shen, X.; Hu, Y.; Xu, K.; Ran, Q.; Yu, Y.; Dai, L.; Yuan, Z.; Huang, L.; Shen, T.; et al. Surface functionalization of titanium implants with chitosan-catechol conjugate for suppression of ros-induced cells damage and improvement of osteogenesis. Biomaterials 2017, 114, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.S.; Wertz, D.L.; Lyons, T.J.; Liou, L.L.; Goto, J.J.; Gralla, E.B. The dark side of dioxygen biochemistry. Curr. Opin. Chem. Biol. 1998, 2, 253–262. [Google Scholar] [CrossRef]
- Rather, H.A.; Thakore, R.; Singh, R.; Jhala, D.; Singh, S.; Vasita, R. Antioxidative study of cerium oxide nanoparticle functionalised PCL-gelatin electrospun fibers for wound healing application. Bioact. Mater. 2018, 3, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I. Oxidative stress, transcription factors and chromatin remodelling in lung inflammation. Biochem. Pharmacol. 2002, 64, 935–942. [Google Scholar] [CrossRef]
- Lacey, D.C.; Simmons, P.J.; Graves, S.E.; Hamilton, J.A. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: Implications for bone repair during inflammation. Osteoarthr. Cartil. 2009, 17, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Tsuzaki, M.; Guyton, G.; Garrett, W.; Archambault, J.M.; Herzog, W.; Almekinders, L.; Bynum, D.; Yang, X.; Banes, A.J. Il-1 beta induces cox2, mmp-1, -3 and -13, adamts-4, il-1 beta and il-6 in human tendon cells. J. Orthop. Res. 2003, 21, 256–264. [Google Scholar] [CrossRef]
- John, T.; Lodka, D.; Kohl, B.; Ertel, W.; Jammrath, J.; Conrad, C.; Stoll, C.; Busch, C.; Schulze-Tanzil, G. Effect of pro-inflammatory and immunoregulatory cytokines on human tenocytes. J. Orthop. Res. 2010, 28, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Song, S.H.; Yun, Y.P.; Choi, B.J.; Kwon, I.K.; Bae, M.S.; Moon, H.J.; Kwon, Y.D. The effect of immobilization of heparin and bone morphogenic protein-2 (bmp-2) to titanium surfaces on inflammation and osteoblast function. Biomaterials 2011, 32, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Thanyacharoen, T.; Chuysinuan, P.; Techasakul, S.; Nooeaid, P.; Ummartyotin, S. Development of a gallic acid-loaded chitosan and polyvinyl alcohol hydrogel composite: Release characteristics and antioxidant activity. Int. J. Biol. Macromol. 2018, 107, 363–370. [Google Scholar] [CrossRef] [PubMed]








| Samples | C1s (%) | N1s (%) | O1s (%) | Total (%) |
|---|---|---|---|---|
| PCL | 77.64 | - | 22.36 | 100 |
| TA/PCL | 76.41 | - | 23.59 | 100 |
| BMP-2/PCL | 76.68 | 0.64 | 22.68 | 100 |
| BMP-2/TA/PCL | 74.49 | 1.28 | 24.23 | 100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Lim, H.; Ahn, J.W.; Jang, D.; Lee, S.H.; Park, K.; Kim, S.E. Design of a 3D BMP-2-Delivering Tannylated PCL Scaffold and Its Anti-Oxidant, Anti-Inflammatory, and Osteogenic Effects In Vitro. Int. J. Mol. Sci. 2018, 19, 3602. https://doi.org/10.3390/ijms19113602
Lee JY, Lim H, Ahn JW, Jang D, Lee SH, Park K, Kim SE. Design of a 3D BMP-2-Delivering Tannylated PCL Scaffold and Its Anti-Oxidant, Anti-Inflammatory, and Osteogenic Effects In Vitro. International Journal of Molecular Sciences. 2018; 19(11):3602. https://doi.org/10.3390/ijms19113602
Chicago/Turabian StyleLee, Jae Yong, Hyunwoong Lim, Jae Won Ahn, Dongsik Jang, Seung Hee Lee, Kyeongsoon Park, and Sung Eun Kim. 2018. "Design of a 3D BMP-2-Delivering Tannylated PCL Scaffold and Its Anti-Oxidant, Anti-Inflammatory, and Osteogenic Effects In Vitro" International Journal of Molecular Sciences 19, no. 11: 3602. https://doi.org/10.3390/ijms19113602
APA StyleLee, J. Y., Lim, H., Ahn, J. W., Jang, D., Lee, S. H., Park, K., & Kim, S. E. (2018). Design of a 3D BMP-2-Delivering Tannylated PCL Scaffold and Its Anti-Oxidant, Anti-Inflammatory, and Osteogenic Effects In Vitro. International Journal of Molecular Sciences, 19(11), 3602. https://doi.org/10.3390/ijms19113602