Uric Acid and Xanthine Levels in Pregnancy Complicated by Gestational Diabetes Mellitus—The Effect on Adverse Pregnancy Outcomes
Abstract
:1. Introduction
2. Results
2.1. Study Subjects and Stratification Criteria
2.2. Comparison of Biochemical and Anthropometrical Data
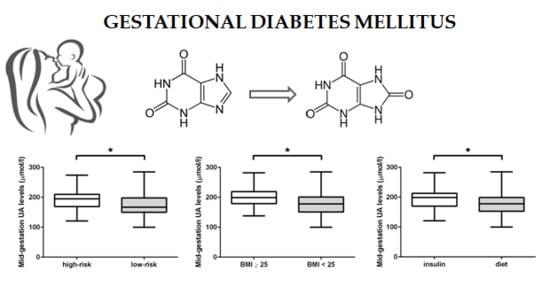
2.3. GDM Sub-Group Analysis
2.4. Labor and Perinatal Outcome Data
2.5. Analyses of Selected SNPs in UA Transporters
3. Discussion
4. Materials and Methods
4.1. Characteristics of Study Subjects
4.2. Blood Sampling, and Uric Acid and Xanthine Measurement
4.3. Genetic Analysis
4.4. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 1-h PPG | 1-h post-load plasma glucose |
| 2-h PPG | 2-h post-load plasma glucose |
| ABCG2 | ATP-binding cassette subfamily G member 2 |
| AMP | adenosine monophosphate |
| ANOVA | analysis of variance |
| AUC | area under the curve |
| BMI | body mass index |
| DIP | diabetes in pregnancy |
| DM | diabetes mellitus |
| DNA | deoxyribonucleic acid |
| EDTA | ethylenediaminetetraacetic acid |
| FPG | fasting plasma glucose |
| GDM | gestational diabetes mellitus |
| GLUT9 | glucose transporter 9 |
| HOMA-IR | homeostatic model assessment for insulin resistance |
| HPLC-UV | high-performance liquid chromatography with ultraviolet detector |
| IQR | interquartile ranges |
| LADA | latent autoimmune diabetes of adults |
| MAF | minor allele frequency |
| MODY | maturity-onset diabetes of the young |
| NS | not significant |
| oGTT | oral glucose tolerance test |
| p | probability |
| SLC2A9 | solute carrier family 2 member 9 |
| SNPs | single-nucleotide polymorphisms |
| T2DM | type 2 diabetes mellitus |
| UA | uric acid |
| WHO | World Health Organization |
| X | xanthine |
| XOR | xanthine oxidoreductase |
References
- Maiuolo, J.; Oppedisano, F.; Gratteri, S.; Muscoli, C.; Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 2016, 213, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.J.; Stewart, C.P.; Hopkins, F.G. On the Anaerobic and Aerobic Oxidation of Xanthin and Hypoxanthin by Tissues and by Milk. Proc. R. Soc. Lond. B Biol. Sci. 1922, 94, 109–131. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Wei, Y.; Liu, M.; Liu, T.; Li, J.; Ji, Y.; Liang, J. Anti-hyperuricemic and nephroprotective effects of Rhizoma Dioscoreae septemlobae extracts and its main component dioscin via regulation of mOAT1, mURAT1 and mOCT2 in hypertensive mice. Arch. Pharm. Res. 2014, 37, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R.; Tyagi, S.C. Uric acid: A new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: The urate redox shuttle. Nutr. Metab. 2004, 1, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onat, A.; Uyarel, H.; Hergenç, G.; Karabulut, A.; Albayrak, S.; Sari, I.; Yazici, M.; Keleş, I. Serum uric acid is a determinant of metabolic syndrome in a population-based study. Am. J. Hypertens. 2006, 19, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Church, T.S.; Meriwether, R.A.; Lobelo, F.; Blair, S.N. Uric acid and the development of metabolic syndrome in women and men. Metabolism 2008, 57, 845–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, A.; Igualá, I.; Bedini, J.; Quintó, L.; Conget, I. Uric acid concentration in subjects at risk of type 2 diabetes mellitus: Relationship to components of the metabolic syndrome. Metabolism 2002, 51, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Ryo, M.; Nakamura, T.; Kihara, S.; Kumada, M.; Shibazaki, S.; Takahashi, M.; Nagai, M.; Matsuzawa, Y.; Funahashi, T. Adiponectin as a biomarker of the metabolic syndrome. Circ. J. 2004, 68, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Köttgen, A.; Albrecht, E.; Teumer, A.; Vitart, V.; Krumsiek, J.; Hundertmark, C.; Pistis, G.; Ruggiero, D.; O’Seaghdha, C.M.; Haller, T.; et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat. Genet. 2013, 45, 145–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Sanna, S.; Maschio, A.; Busonero, F.; Usala, G.; Mulas, A.; Lai, S.; Dei, M.; Orrù, M.; Albai, G.; et al. The GLUT9 gene is associated with serum uric acid levels in Sardinia and Chianti cohorts. PLoS Genet. 2007, 3, e194. [Google Scholar] [CrossRef] [PubMed]
- Döring, A.; Gieger, C.; Mehta, D.; Gohlke, H.; Prokisch, H.; Coassin, S.; Fischer, G.; Henke, K.; Klopp, N.; Kronenberg, F.; et al. SLC2A9 influences uric acid concentrations with pronounced sex-specific effects. Nat. Genet. 2008, 40, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Woodward, O.M.; Köttgen, A.; Coresh, J.; Boerwinkle, E.; Guggino, W.B.; Köttgen, M. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc. Natl. Acad. Sci. USA 2009, 106, 10338–10342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiefari, E.; Arcidiacono, B.; Foti, D.; Brunetti, A. Gestational diabetes mellitus: An updated overview. J. Endocrinol. Investig. 2017, 40, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Ehrenberg, H.M. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG 2006, 113, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Kaaja, R.; Rönnemaa, T. Gestational diabetes: Pathogenesis and consequences to mother and offspring. Rev. Diabet. Stud. 2008, 5, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Group, H.S.C.R. The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Int. J. Gynaecol. Obstet. 2002, 78, 69–77. [Google Scholar]
- Retnakaran, R.; Qi, Y.; Connelly, P.W.; Sermer, M.; Zinman, B.; Hanley, A.J. Glucose intolerance in pregnancy and postpartum risk of metabolic syndrome in young women. J. Clin. Endocrinol. Metab. 2010, 95, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Bodnar, L.M.; Lain, K.Y.; Hubel, C.A.; Markovic, N.; Ness, R.B.; Powers, R.W. Uric acid is as important as proteinuria in identifying fetal risk in women with gestational hypertension. Hypertension 2005, 46, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.M.; Chow, S.N.; Huang, H.C.; Hsieh, F.J.; Chen, H.Y.; Lee, T.Y.; Ouyang, P.C.; Chen, Y.P. The placental transfer and concentration difference in maternal and neonatal serum uric acid at parturition: Comparison of normal pregnancies and gestosis. Biol. Res. Pregnancy Perinatol. 1987, 8, 35–39. [Google Scholar] [PubMed]
- Rasika, C.; Samal, S.; Ghose, S. Association of Elevated first Trimester Serum Uric Acid Levels with Development of GDM. J. Clin. Diagn. Res. 2014, 8, OC01–OC05. [Google Scholar] [CrossRef]
- Wolak, T.; Sergienko, R.; Wiznitzer, A.; Paran, E.; Sheiner, E. High uric acid level during the first 20 weeks of pregnancy is associated with higher risk for gestational diabetes mellitus and mild preeclampsia. Hypertens. Pregnancy 2012, 31, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Şahin Aker, S.; Yüce, T.; Kalafat, E.; Seval, M.; Söylemez, F. Association of first trimester serum uric acid levels gestational diabetes mellitus development. Turk. J. Obstet. Gynecol. 2016, 13, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Güngör, E.S.; Danişman, N.; Mollamahmutoğlu, L. Relationship between serum uric acid, creatinine, albumin and gestational diabetes mellitus. Clin. Chem. Lab. Med. 2006, 44, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Laughon, S.K.; Catov, J.; Roberts, J.M. Uric acid concentrations are associated with insulin resistance and birthweight in normotensive pregnant women. Am. J. Obstet. Gynecol. 2009, 201, 582.e1–582.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bainbridge, S.A.; Roberts, J.M. Uric acid as a pathogenic factor in preeclampsia. Placenta 2008, 29 (Suppl. A), S67–S72. [Google Scholar] [CrossRef]
- Laughon, S.K.; Catov, J.; Powers, R.W.; Roberts, J.M.; Gandley, R.E. First trimester uric acid and adverse pregnancy outcomes. Am. J. Hypertens. 2011, 24, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Livingston, J.R.; Payne, B.; Brown, M.; Roberts, J.M.; Côté, A.M.; Magee, L.A.; von Dadelszen, P.; Group, P.S. Uric Acid as a predictor of adverse maternal and perinatal outcomes in women hospitalized with preeclampsia. J. Obstet. Gynaecol. Can. 2014, 36, 870–877. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, A.K.; Maini, B. Impact of maternal serum uric acid on perinatal outcome in women with hypertensive disorders of pregnancy: A prospective study. Pregnancy Hypertens. 2017, 10, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Thangaratinam, S.; Ismail, K.M.; Sharp, S.; Coomarasamy, A.; Khan, K.S. Accuracy of serum uric acid in predicting complications of pre-eclampsia: A systematic review. BJOG 2006, 113, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zablith, N.R.; Burns, D.H.; Skinner, C.D.; Koski, K.G. Second trimester amniotic fluid transferrin and uric acid predict infant birth outcomes. Prenat. Diagn. 2008, 28, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Nasri, K.; Razavi, M.; Rezvanfar, M.R.; Mashhadi, E.; Chehrei, A.; Mohammadbeigi, A. Mid-gestational serum uric acid concentration effect on neonate birth weight and insulin resistance in pregnant women. Int. J. Crit. Illn. Inj. Sci. 2015, 5, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; Wang, L.; Wang, J.; Li, W.; Liu, H.; Zhang, S.; Li, L.; Tian, H.; Xun, P.; Yang, X.; et al. Uric acid and diabetes risk among Chinese women with a history of gestational diabetes mellitus. Diabetes Res. Clin. Pract. 2017, 134, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Corrado, F.; Pintaudi, B.; D'Anna, R.; Santamaria, A.; Giunta, L.; Di Benedetto, A. Perinatal outcome in a Caucasian population with gestational diabetes and preexisting diabetes first diagnosed in pregnancy. Diabetes Metab. 2016, 42, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Abbassi-Ghanavati, M.; Greer, L.G.; Cunningham, F.G. Pregnancy and laboratory studies: A reference table for clinicians. Obstet. Gynecol. 2009, 114, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Simmonds, H.A. Purines: Basic and Clinical Aspects; Kluwer Academic: Dordrecht, The Netherlands; London, UK, 1991; 257p. [Google Scholar]
- Fang, P.; Li, X.; Luo, J.J.; Wang, H.; Yang, X.F. A Double-edged Sword: Uric Acid and Neurological Disorders. Brain Disord. Ther. 2013, 2, 109. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.Y.; Johnson, R.J. Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, W.; McRae, S.; Marek, G.; Wymer, D.; Pannu, V.; Baylis, C.; Johnson, R.J.; Sautin, Y.Y. Hyperuricemia as a mediator of the proinflammatory endocrine imbalance in the adipose tissue in a murine model of the metabolic syndrome. Diabetes 2011, 60, 1258–1269. [Google Scholar] [CrossRef] [PubMed]
- Meshkani, R.; Zargari, M.; Larijani, B. The relationship between uric acid and metabolic syndrome in normal glucose tolerance and normal fasting glucose subjects. Acta Diabetol. 2011, 48, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Dogan, A.; Yarlioglues, M.; Kaya, M.G.; Karadag, Z.; Dogan, S.; Ardic, I.; Dogdu, O.; Kilinc, Y.; Zencir, C.; Akpek, M.; et al. Effect of long-term and high-dose allopurinol therapy on endothelial function in normotensive diabetic patients. Blood Press. 2011, 20, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Nakagawa, T.; Sanchez-Lozada, L.G.; Shafiu, M.; Sundaram, S.; Le, M.; Ishimoto, T.; Sautin, Y.Y.; Lanaspa, M.A. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 2013, 62, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.Y.; Nakagawa, T.; Zharikov, S.; Johnson, R.J. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am. J. Physiol. Cell Physiol. 2007, 293, C584–C596. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, Y.; Nishizawa, H.; Tochino, Y.; Nakatsuji, H.; Sekimoto, R.; Nagao, H.; Shirakura, T.; Kato, K.; Imaizumi, K.; Takahashi, H.; et al. Uric acid secretion from adipose tissue and its increase in obesity. J. Biol. Chem. 2013, 288, 27138–27149. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.J.; Tzameli, I.; Pissios, P.; Rovira, I.; Gavrilova, O.; Ohtsubo, T.; Chen, Z.; Finkel, T.; Flier, J.S.; Friedman, J.M. Xanthine oxidoreductase is a regulator of adipogenesis and PPARgamma activity. Cell Metab. 2007, 5, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Fruehwald-Schultes, B.; Peters, A.; Kern, W.; Beyer, J.; Pfützner, A. Serum leptin is associated with serum uric acid concentrations in humans. Metabolism 1999, 48, 677–680. [Google Scholar] [CrossRef]
- Bedir, A.; Topbas, M.; Tanyeri, F.; Alvur, M.; Arik, N. Leptin might be a regulator of serum uric acid concentrations in humans. Jpn. Heart J. 2003, 44, 527–536. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.P.; Burini, R.C. High plasma uric acid concentration: Causes and consequences. Diabetol. Metab. Syndr. 2012, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, F.; Yamashita, S.; Nakamura, T.; Nishida, M.; Nozaki, S.; Funahashi, T.; Matsuzawa, Y. Effect of visceral fat accumulation on uric acid metabolism in male obese subjects: Visceral fat obesity is linked more closely to overproduction of uric acid than subcutaneous fat obesity. Metabolism 1998, 47, 929–933. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Cicerchi, C.; Garcia, G.; Li, N.; Roncal-Jimenez, C.A.; Rivard, C.J.; Hunter, B.; Andrés-Hernando, A.; Ishimoto, T.; Sánchez-Lozada, L.G.; et al. Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver. PLoS ONE 2012, 7, e48801. [Google Scholar] [CrossRef] [PubMed]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef] [PubMed]
- Molęda, P.; Fronczyk, A.; Safranow, K.; Majkowska, L. Is Uric Acid a Missing Link between Previous Gestational Diabetes Mellitus and the Development of Type 2 Diabetes at a Later Time of Life? PLoS ONE 2016, 11, e0154921. [Google Scholar] [CrossRef] [PubMed]
- Modan, M.; Halkin, H.; Karasik, A.; Lusky, A. Elevated serum uric acid—A facet of hyperinsulinaemia. Diabetologia 1987, 30, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, L.D.; Costantine, M.M.; Hankins, G.D.V. 2—Physiologic Changes During Pregnancy A2—Mattison, Donald R. In Clinical Pharmacology During Pregnancy; Academic Press: Cambridge, MA, USA, 2013; pp. 5–16. [Google Scholar]
- Hung, T.H.; Lo, L.M.; Chiu, T.H.; Li, M.J.; Yeh, Y.L.; Chen, S.F.; Hsieh, T.T. A longitudinal study of oxidative stress and antioxidant status in women with uncomplicated pregnancies throughout gestation. Reprod. Sci. 2010, 17, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Vitart, V.; Rudan, I.; Hayward, C.; Gray, N.K.; Floyd, J.; Palmer, C.N.; Knott, S.A.; Kolcic, I.; Polasek, O.; Graessler, J.; et al. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat. Genet. 2008, 40, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.C.; Hung, C.C.; Chen, S.C.; Lin, M.Y.; Chen, L.I.; Hwang, D.Y.; Chang, J.M.; Tsai, J.C.; Chen, H.C.; Hwang, S.J. The rs1014290 polymorphism of the SLC2A9 gene is associated with type 2 diabetes mellitus in Han Chinese. Exp. Diabetes Res. 2011, 2011, 527520. [Google Scholar] [CrossRef] [PubMed]
- Pleskacova, A.; Brejcha, S.; Pacal, L.; Kankova, K.; Tomandl, J. Simultaneous Determination of Uric Acid, Xanthine and Hypoxanthine in Human Plasma and Serum by HPLC–UV: Uric Acid Metabolism Tracking. Chromatographia 2016, 80, 529–536. [Google Scholar] [CrossRef]

| Parameter | GDM (n = 109) | Controls (n = 34) | p |
|---|---|---|---|
| Age (years) | 33 (30–35) | 31 (28–33) | 0.040 |
| Pre-gestational BMI (kg m-2) | 24.6 (21.2–28.7) | 22.5 (20.6–27.0) | NS |
| Weight gain during pregnancy (kg) | 8 (5–10) | 13 (10–17) | <1 × 10−6 |
| Offspring birth weight (g) | 3120 (2790–3500) | 3355 (3070–3700) | 0.032 |
| Primiparity | 38.5% | 44.1% | NS |
| History of previous GDM | 26.2% | 0% | 0.009 |
| Family history of DM | 72.1% | 27.3% | <1 × 10−4 |
| FPG (mmol/L) mid-gestation | 4.8 (4.4–5.2) | 4.2 (4.0–4.4) | <1 × 10−6 |
| 1-h PPG (mmol/L) mid-gestation | 9.4 (8.9–10.1) | 5.9 (5.3–6.5) | <1 × 10−6 |
| 2-h PPG (mmol/L) mid-gestation | 8.2 (7.7–8.8) | 5.5 (4.8–6.1) | <1 × 10−6 |
| AUC (mmol/L/h) mid-gestation | 13.0 (12.3–13.7) | 9.6 (8.9–10.3) | <1 × 10−6 |
| FPG (mmol/L) postpartum | 4.7 (4.4–4.9) | - | - |
| 1-h PPG (mmol/L) postpartum | 6.3 (5.1–7.8) | - | - |
| 2-h PPG (mmol L-1) postpartum | 5.0 (4.3–5.9) | - | - |
| AUC (mmol/L/h) postpartum | 9.7 (8.8–10.6) | - | - |
| X levels (μmol/L) mid-gestation | 3.61 (3.41–3.85) | 3.41 (3.21–3.70) | 0.049 |
| X levels (μmol/L) postpartum | 4.14 (3.91–4.61) | 3.79 (3.53–4.20) | 0.0002 |
| UA levels (μmol/L) mid-gestation | 183 (154–205) | 178 (168–190) | NS |
| UA levels (μmol/L) postpartum | 221(195–249) | 213 (190–248) | NS |
| Parameter | GDM (n = 57) | Controls (n = 34) | p |
|---|---|---|---|
| Primiparity | 57.9% | 44.1% | NS |
| Gestational age at delivery (weeks) | 40 (39–40) | 40 (39–40.5) | NS |
| Premature labor | 12.3% | 0% | NS |
| Spontaneous onset of labor | 54.4% | 66.0% | NS |
| Protracted labor (>480 min) | 3.51% | 4.55% | NS |
| Caesarean section rate | 33.3% | 13.6% | 0.043 |
| Complications following childbirth | 5.26% | 4.55% | NS |
| Pathological Apgar score | 5.26% | 0% | NS |
| Pathological umbilical cord-blood pH | 1.75% | 4.55% | NS |
| Pathological umbilical cord base excess | 3.51% | 0% | NS |
| UA transporter (gene) | SNP | Nucleotide Substitution | SNP Effect (Position) | MAF in GDM Group (%) | MAF in Control Group (%) | p (Genotype and Allele Frequency) |
|---|---|---|---|---|---|---|
| ABCG2 (ABCG2) | rs2231142 | G/T | 141 Q/K (exon) | T 10.5 | T 4.69 | both NS |
| GLUT9 (SLC2A9) | rs1014290 | A/G | (intron) | G 35.6 | G 29.3 | both NS |
| rs12498742 | A/G | (intron) | G 29.5 | G 37.9 * | both NS | |
| rs16890979 | C/T | 253 V/I (exon) | T 26.6 | T 47.9 | both NS | |
| rs734553 | G/T | (intron) | G 28.0 | G29.0 | both NS |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pleskacova, A.; Bartakova, V.; Chalasova, K.; Pacal, L.; Kankova, K.; Tomandl, J. Uric Acid and Xanthine Levels in Pregnancy Complicated by Gestational Diabetes Mellitus—The Effect on Adverse Pregnancy Outcomes. Int. J. Mol. Sci. 2018, 19, 3696. https://doi.org/10.3390/ijms19113696
Pleskacova A, Bartakova V, Chalasova K, Pacal L, Kankova K, Tomandl J. Uric Acid and Xanthine Levels in Pregnancy Complicated by Gestational Diabetes Mellitus—The Effect on Adverse Pregnancy Outcomes. International Journal of Molecular Sciences. 2018; 19(11):3696. https://doi.org/10.3390/ijms19113696
Chicago/Turabian StylePleskacova, Anna, Vendula Bartakova, Katarina Chalasova, Lukas Pacal, Katerina Kankova, and Josef Tomandl. 2018. "Uric Acid and Xanthine Levels in Pregnancy Complicated by Gestational Diabetes Mellitus—The Effect on Adverse Pregnancy Outcomes" International Journal of Molecular Sciences 19, no. 11: 3696. https://doi.org/10.3390/ijms19113696
APA StylePleskacova, A., Bartakova, V., Chalasova, K., Pacal, L., Kankova, K., & Tomandl, J. (2018). Uric Acid and Xanthine Levels in Pregnancy Complicated by Gestational Diabetes Mellitus—The Effect on Adverse Pregnancy Outcomes. International Journal of Molecular Sciences, 19(11), 3696. https://doi.org/10.3390/ijms19113696