Blood Outgrowth and Proliferation of Endothelial Colony Forming Cells are Related to Markers of Disease Severity in Patients with Pulmonary Arterial Hypertension
Abstract
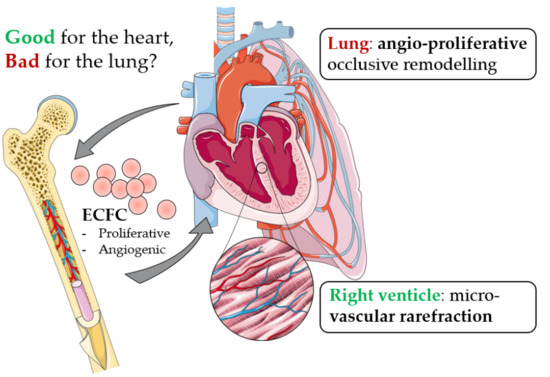
:1. Introduction
2. Results
2.1. PAH Patient Characteristics and ECFC Outgrowth
2.2. Quantification of PAH ECFC Proliferation and Tube Formation
2.2.1. Proliferation
2.2.2. Relation to Markers of RV Function and Lung PVR
2.2.3. Tube Formation
2.3. Transplantation of Highly Proliferative PAH ECFC with Tube Formation Ability in an Animal Model with Chronic RV-Failure
3. Discussion
4. Materials and Methods
4.1. Inclusion
4.2. Acquisition of Hemodynamic Parameters and RV Volume Measurements in PAH Patients
4.3. Time to Clinical Worsening
4.4. ECFC Isolation, Culture and Characterization
4.5. Proliferation Assay
4.6. Tube Formation Assay
4.7. Pulmonary Trunk Banding (PTB) in Rats
4.8. Transplantation of PAH ECFC
4.9. Acquisition of Hemodynamic Parameters and RV Volume Measurements in PTB-Animals
4.10. RV-Small Vessel Density
4.11. Statistics
4.12. Handling of Missing Data
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Voelkel, N.F.; Gomez-Arroyo, J.G.; Abbate, A.; Bogaard, H.J.; Nicolls, M.R. Pathobiology of pulmonary arterial hypertension and right ventricular failure. Eur. Respir J. 2012, 40, 1555–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guignabert, C.; Dorfmuller, P. Pathology and pathobiology of pulmonary hypertension. Semin. Respir. Crit. Care Med. 2013, 34, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Vonk-Noordegraaf, A.; Haddad, F.O.; Chin, K.M.; Forfia, P.R.; Kawut, S.M.; Lumens, J.; Naeije, R.; Newman, J.; Oudiz, R.J.; Provencher, S.; et al. Right Heart Adaptation to Pulmonary Arterial-Hypertension: Physiology and Pathobiology. J. Am. Coll. Cardiol. 2013, 62, D22–D33. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arroyo, J.; Sandoval, J.; Simon, M.A.; Dominguez-Cano, E.; Voelkel, N.F.; Bogaard, H.J. Treatment for pulmonary arterial hypertension-associated right ventricular dysfunction. Ann. Am. Thorac. Soc. 2014, 11, 1101–1115. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.A.; Erzurum, S.; Asosingh, K. Biology and flow cytometry of proangiogenic hematopoietic progenitors cells. Cytometry A 2015, 87, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.A.; Mead, L.E.; Tanaka, H.; Meade, V.; Fenoglio, A.; Mortell, K.; Pollok, K.; Ferkowicz, M.J.; Gilley, D.; Yoder, M.C. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood 2004, 104, 2752–2760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, J.; Li, J.; Orschell, C.M.; March, K.L. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 2003, 107, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tao, Y.; Ren, S.; Liu, H.; Zhou, H.; Hu, J.; Tang, Y.; Zhang, B.; Chen, H. Isolation and characterization of human umbilical cord-derived endothelial colony-forming cells. Exp. Ther. Med. 2017, 14, 4160–4166. [Google Scholar] [CrossRef] [PubMed]
- Ikutomi, M.; Sahara, M.; Nakajima, T.; Minami, Y.; Morita, T.; Hirata, Y.; Komuro, I.; Nakamura, F.; Sata, M. Diverse contribution of bone marrow-derived late-outgrowth endothelial progenitor cells to vascular repair under pulmonary arterial hypertension and arterial neointimal formation. J. Mol. Cell. Cardiol. 2015, 86, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Muller, P.; Beltrami, A.P.; Cesselli, D.; Pfeiffer, P.; Kazakov, A.; Bohm, M. Myocardial regeneration by endogenous adult progenitor cells. J. Mol. Cell. Cardiol. 2005, 39, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.M.; Valgimigli, M.; Giannico, M.B.; Zaccone, V.; Perfetti, M.; D’Amario, D.; Rebuzzi, A.G.; Crea, F. From bone marrow to the arterial wall: The ongoing tale of endothelial progenitor cells. Eur. Heart J. 2009, 30, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Asosingh, K.; Aldred, M.A.; Vasanji, A.; Drazba, J.; Sharp, J.; Farver, C.; Comhair, S.A.; Xu, W.; Licina, L.; Huang, L.; et al. Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension. Am. J. Pathol. 2008, 172, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Toshner, M.; Voswinckel, R.; Southwood, M.; Al-Lamki, R.; Howard, L.S.; Marchesan, D.; Yang, J.; Suntharalingam, J.; Soon, E.; Exley, A.; et al. Evidence of dysfunction of endothelial progenitors in pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2009, 180, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.H.; Wang, X.X.; Gu, G.S.; Shang, Y.P.; Zhang, F.R.; Chen, J.Z. Reduced number and activity of circulating endothelial progenitor cells in patients with idiopathic pulmonary arterial hypertension. Respir. Med. 2008, 102, 1073–1079. [Google Scholar] [CrossRef]
- Diller, G.P.; van Eijl, S.; Okonko, D.O.; Howard, L.S.; Ali, O.; Thum, T.; Wort, S.J.; Bedard, E.; Gibbs, J.S.; Bauersachs, J.; et al. Circulating endothelial progenitor cells in patients with Eisenmenger syndrome and idiopathic pulmonary arterial hypertension. Circulation 2008, 117, 3020–3030. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.H.; Wang, X.X.; Zhang, F.R.; Shang, Y.P.; Tao, Q.M.; Zhu, J.H.; Chen, J.Z. Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: Open-label pilot study. Pediatr. Transplant. 2008, 12, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Zhang, F.R.; Shang, Y.P.; Zhu, J.H.; Xie, X.D.; Tao, Q.M.; Zhu, J.H.; Chen, J.Z. Transplantation of Autologous Endothelial Progenitor Cells May Be Beneficial in Patients with Idiopathic Pulmonary Arterial Hypertension: A Pilot Randomized Controlled Trial. J. Am. Coll. Cardiol. 2007, 49, 1566–1571. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Nakamura, T.; Toba, T.; Kajiwara, N.; Kato, H.; Shimizu, Y. Transplantation of endothelial progenitor cells into the lung to alleviate pulmonary hypertension in dogs. Tissue Eng. 2004, 10, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Yip, H.K.; Chang, L.T.; Sun, C.K.; Sheu, J.J.; Chiang, C.H.; Youssef, A.A.; Lee, F.Y.; Wu, C.J.; Fu, M. Autologous transplantation of bone marrow-derived endothelial progenitor cells attenuates monocrotaline-induced pulmonary arterial hypertension in rats. Crit. Care Med. 2008, 36, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Fu, G.S.; Yang, J.X.; Zhang, F.R.; Wang, X.X. Endothelial progenitor cells may inhibit apoptosis of pulmonary microvascular endothelial cells: New insights into cell therapy for pulmonary arterial hypertension. Cytotherapy 2009, 11, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Marsboom, G.; Pokreisz, P.; Gheysens, O.; Vermeersch, P.; Gillijns, H.; Pellens, M.; Liu, X.; Collen, D.; Janssens, S. Sustained endothelial progenitor cell dysfunction after chronic hypoxia-induced pulmonary hypertension. Stem Cells 2008, 26, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Aliotta, J.M.; Pereira, M.; Wen, S.; Dooner, M.S.; Del Tatto, M.; Papa, E.; Cheng, Y.; Goldberg, L.; Quesenberry, P.J.; Klinger, J. Bone Marrow Endothelial Progenitor Cells Are the Cellular Mediators of Pulmonary Hypertension in the Murine Monocrotaline Injury Model. Am. J. Respir. Crit. Care 2017, 6, 1595–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.D.; Courtman, D.W.; Deng, Y.; Kugathasan, L.; Zhang, Q.; Stewart, D.J. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: Efficacy of combined cell and eNOS gene therapy in established disease. Circ. Res. 2005, 96, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhu, W.; Xia, L.M.; Yang, Y.; Liu, H.; Shen, J.Q.; Zhu, J.S.; Xu, Y.W.; Yang, Z.H.; Wang, C.S. Therapeutic effect of eNOS-transfected endothelial progenitor cells on hemodynamic pulmonary arterial hypertension. Hypertens. Res. 2013, 36, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Siegel, G.; Fleck, E.; Elser, S.; Hermanutz-Klein, U.; Waidmann, M.; Northoff, H.; Seifried, E.; Schafer, R. Manufacture of endothelial colony-forming progenitor cells from steady-state peripheral blood leukapheresis using pooled human platelet lysate. Transfusion 2018, 58, 1132–1142. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Solomon, M.A.; McCoy, J.P., Jr. Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytom. B Clin. Cytom. 2005, 64, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolbe, M.; Dohle, E.; Katerla, D.; Kirkpatrick, C.J.; Fuchs, S. Enrichment of Outgrowth Endothelial Cells in High and Low Colony-Forming Cultures from Peripheral Blood Progenitors. Tissue Eng. Part C-Method 2010, 16, 877–886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasev, D.; van Wijhe, M.H.; Weijers, E.M.; van Hinsbergh, V.W.; Koolwijk, P. Long-Term Expansion in Platelet Lysate Increases Growth of Peripheral Blood-Derived Endothelial-Colony Forming Cells and Their Growth Factor-Induced Sprouting Capacity. PLoS ONE 2015, 10, e0129935. [Google Scholar] [CrossRef] [PubMed]
- Hjalmarsson, C.; Radegran, G.; Kylhammar, D.; Rundqvist, B.; Multing, J.; Nisell, M.D.; Kjellstrom, B.; SveFPH and SPAHR. Impact of age and comorbidity on risk stratification in idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2018, 51, 1702310. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Huscher, D.; Ghofrani, H.A.; Delcroix, M.; Distler, O.; Schweiger, C.; Grunig, E.; Staehler, G.; Rosenkranz, S.; Halank, M.; et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: Results from the COMPERA registry. Int. J. Cardiol. 2013, 168, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Shahrivari, M.; Wise, E.; Resende, M.; Shuster, J.J.; Zhang, J.; Bolli, R.; Cooke, J.P.; Hare, J.M.; Henry, T.D.; Khan, A.; et al. Peripheral Blood Cytokine Levels After Acute Myocardial Infarction IL-1 beta- and IL-6-Related Impairment of Bone Marrow Function. Circ. Res. 2017, 120, 1947–1957. [Google Scholar] [CrossRef] [PubMed]
- Harbaum, L.; Renk, E.; Yousef, S.; Glatzel, A.; Luneburg, N.; Hennigs, J.K.; Oqueka, T.; Baumann, H.J.; Atanackovic, D.; Grunig, E.; et al. Acute effects of exercise on the inflammatory state in patients with idiopathic pulmonary arterial hypertension. BMC Pulm. Med. 2016, 16, 145. [Google Scholar] [CrossRef] [PubMed]
- Assmus, B.; Schachinger, V.; Teupe, C.; Britten, M.; Lehmann, R.; Dobert, N.; Grunwald, F.; Aicher, A.; Urbich, C.; Martin, H.; et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction-(TOPCARE-AMI). Circulation 2002, 106, 3009–3017. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Walker, L.; Afentoulis, M.; Anderson, D.A.; Jauron-Mills, L.; Corless, C.L.; Fleming, W.H. Transplanted human bone marrow contributes to vascular endothelium. Blood 2004, 104, 733a–734a. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Sharpe, E.E.; Maupin, A.B.; Teleron, A.A.; Pyle, A.L.; Carmeliet, P.; Young, P.P. VEGF and PlGF promote adult vasculogenesis by enhancing EPC recruitment and vessel formation at the site of tumor neovascularization. FASEB J. 2006, 20, 1495–1497. [Google Scholar] [CrossRef] [PubMed]
- Shintani, S.; Murohara, T.; Ikeda, H.; Ueno, T.; Honma, T.; Katoh, A.; Sasaki, K.I.; Shimada, T.; Oike, Y.; Imaizumi, T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation 2001, 103, 2776–2779. [Google Scholar] [CrossRef] [PubMed]
- Kissel, C.K.; Lehmann, R.; Assmus, B.; Aicher, A.; Honold, J.; Fischer-Rasokat, U.; Heeschen, C.; Spyridopoulos, I.; Dimmeler, S.; Zeiher, A.M. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J. Am. Coll. Cardiol. 2007, 49, 2341–2349. [Google Scholar] [CrossRef] [PubMed]
- Groth, A.; Vrugt, B.; Brock, M.; Speich, R.; Ulrich, S.; Huber, L.C. Inflammatory cytokines in pulmonary hypertension. Respir. Res. 2014, 15, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, X.Y.; Mo, Z.H.; Chen, K.; He, H.H.; Xie, Y.H. Glucagon-like Peptide-1 improves proliferation and differentiation of endothelial progenitor cells via upregulating VEGF generation. Med. Sci. Monit. 2011, 17, Br35–Br41. [Google Scholar] [CrossRef]
- Yang, L.; Yang, X.C.; Yang, J.K.; Guo, Y.H.; Yi, F.F.; Fan, Q.; Liu, X.L. Cyclosporin A suppresses proliferation of endothelial progenitor cells: Involvement of nitric oxide synthase inhibition. Intern. Med. 2008, 47, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, X.G.; Du, C.Q.; Yang, J.X.; Jiang, D.M.; Li, B.; Zhou, W.J.; Zhang, F.R. Interleukin-1 Beta Increases Activity of Human Endothelial Progenitor Cells: Involvement of PI3K-Akt Signaling Pathway. Inflammation 2012, 35, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.G.; Zhong, Z.Y.; Sun, G.F.; Zhou, Y.X.; Zhao, Y. Effects of tumour necrosis factor-alpha on activity and nitric oxide synthase of endothelial progenitor cells from peripheral blood. Cell Prolif. 2011, 44, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Lindner, J.; Maruna, P.; Kunstyr, J.; Jansa, P.; Gurlich, R.; Kubzova, K.; Zakharchenko, M.; Linhart, A. Hemodynamic instability after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension correlates with cytokine network hyperstimulation. Eur. Surg. Res. 2009, 43, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Dolenc, J.; Sebestjen, M.; Vrtovec, B.; Kozelj, M.; Haddad, F. Pulmonary hypertension in patients with advanced heart failure is associated with increased levels of interleukin-6. Biomarkers 2014, 19, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Natanson, C.; Eichenholz, P.W.; Danner, R.L.; Eichacker, P.Q.; Hoffman, W.D.; Kuo, G.C.; Banks, S.M.; MacVittie, T.J.; Parrillo, J.E. Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J. Exp. Med. 1989, 169, 823–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuccuini, W.; Poitevin, S.; Poitevin, G.; Dignat-George, F.; Cornillet-Lefebvre, P.; Sabatier, F.; Nguyen, P. Tissue factor up-regulation in proinflammatory conditions confers thrombin generation capacity to endothelial colony-forming cells without influencing non-coagulant properties in vitro. J. Thromb. Haemost. 2010, 8, 2042–2052. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.H.; Chen, Y.H.; Tsai, H.Y.; Chen, J.S.; Wu, T.C.; Lin, F.Y.; Sata, M.; Chen, J.W.; Lin, S.J. Intake of Red Wine Increases the Number and Functional Capacity of Circulating Endothelial Progenitor Cells by Enhancing Nitric Oxide Bioavailability. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 869–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montani, D.; Perros, F.; Gambaryan, N.; Girerd, B.; Dorfmuller, P.; Price, L.C.; Huertas, A.; Hammad, H.; Lambrecht, B.N.; Simonneau, G.; et al. C-Kit-Positive Cells Accumulate in Remodeled Vessels of Idiopathic Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care 2011, 184, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Gambaryan, N.; Perros, F.; Montani, D.; Cohen-Kaminsky, S.; Mazmanian, M.; Renaud, J.F.; Simonneau, G.; Lombet, A.; Humbert, M. Targeting of c-kit+ hematopoietic progenitor cells prevents hypoxic pulmonary hypertension. Eur. Respir. J. 2010, 37, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Farkas, D.; Kraskauskas, D.; Drake, J.I.; Alhussaini, A.A.; Kraskauskiene, V.; Bogaard, H.J.; Cool, C.D.; Voelkel, N.F.; Farkas, L. CXCR4 Inhibition Ameliorates Severe Obliterative Pulmonary Hypertension and Accumulation of C-Kit(+) Cells in Rats. PLoS ONE 2014, 9, e89810. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Fadini, G.P.; Lunardi, F.; Agostini, C.; Boscaro, E.; Calabrese, F.; Marulli, G.; Rea, F. Increased tissue endothelial progenitor cells in end-stage lung diseases with pulmonary hypertension. J. Heart Lung Transplant. 2012, 31, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, T.; Naito, H.; Suehiro, J.; Lin, Y.; Kawaji, H.; Iba, T.; Kouno, T.; Ishikawa-Kato, S.; Furuno, M.; Takara, K.; et al. CD157 Marks Tissue-Resident Endothelial Stem Cells with Homeostatic and Regenerative Properties. Cell Stem Cell 2018, 22, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Tomanek, R.J. Response of the coronary vasculature to myocardial hypertrophy. J. Am. Coll. Cardiol. 1990, 15, 528–533. [Google Scholar] [CrossRef]
- Vogel-Claussen, J.; Skrok, J.; Shehata, M.L.; Singh, S.; Sibley, C.T.; Boyce, D.M.; Lechtzin, N.; Girgis, R.E.; Mathai, S.C.; Goldstein, T.A.; et al. Right and left ventricular myocardial perfusion reserves correlate with right ventricular function and pulmonary hemodynamics in patients with pulmonary arterial hypertension. Radiology 2011, 258, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Van Wolferen, S.A.; Marcus, J.T.; Westerhof, N.; Spreeuwenberg, M.D.; Marques, K.M.; Bronzwaer, J.G.; Henkens, I.R.; Gan, C.T.; Boonstra, A.; Postmus, P.E.; et al. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur. Heart J. 2008, 29, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Spruijt, O.A.; Bogaard, H.J.; Vonk-Noordegraaf, A. Assessment of right ventricular responses to therapy in pulmonary hypertension. Drug Discov. Today 2014, 19, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Zeisberger, S.M.; Zoller, S.; Riegel, M.; Chen, S.; Krenning, G.; Harmsen, M.C.; Sachinidis, A.; Zisch, A.H. Optimization of the culturing conditions of human umbilical cord blood-derived endothelial colony-forming cells under xeno-free conditions applying a transcriptomic approach. Genes Cells 2010, 15, 671–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasev, D.; Konijnenberg, L.S.; Amado-Azevedo, J.; van Wijhe, M.H.; Koolwijk, P.; van Hinsbergh, V.W. CD34 expression modulates tube-forming capacity and barrier properties of peripheral blood-derived endothelial colony-forming cells (ECFCs). Angiogenesis 2016, 19, 325–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, S.; Schultz, J.G.; Andersen, A.; Ringgaard, S.; Nielsen, J.M.; Holmboe, S.; Vildbrad, M.D.; de Man, F.S.; Bogaard, H.J.; Vonk-Noordegraaf, A.; et al. Effects of bisoprolol and losartan treatment in the hypertrophic and failing right heart. J. Card. Fail. 2014, 20, 864–873. [Google Scholar] [CrossRef] [PubMed]




| ECFC Outgrowth | No ECFC Outgrowth | p | |
|---|---|---|---|
| Patient characteristics | |||
| n= | 21 | 12 | |
| Diagnosis (n) | iPAH(10), hPAH(5), PAH-CTD(3), PAH-CHD(2) | iPAH(7), hPAH(1), PAH-CTD(3), PAH-CHD(1) | |
| Age (yrs) | 45 ± 12 | 56 ± 16 | 0.036 * |
| % female | 90% | 83% | |
| Disease duration (yrs) | 4.1 (0.3–9.3) | 2.7 (0.4–9.0) | 0.687 |
| Treatment PGI iv/sc (%) | 52% | 25% | 0.122 |
| Laboratory tests | |||
| NT-proBNP | 1296 (231–4441) | 363 (154–888) | 0.172 |
| Hemodynamics | |||
| mPAP (mmHg) | 47 ± 18 | 47 ± 10 | 0.956 |
| PVR (dynes.s.cm−5) | 600 (383–928) | 564 (214–882) | 0.304 |
| CO (l/min) | 4.9 (4.1–6.1) | 5.6 (3.8–9.0) | 0.092 |
| SvO2 (%) | 63 (58–73) | 72 (62–80) | 0.037 * |
| Cardiac function | |||
| RVEDV (mL) | 171 (113–291) | 145 (77–165) | 0.238 |
| RVEF (%) | 29 (10–51) | 46 (30-60) | 0.037 * |
| Performance | |||
| 6MWT (m) | 386 ± 156 | 381 ± 110 | 0.925 |
| Heart failure acc. to NYHA | 3.0 (2.0–3.5) | 2.5 (2.0–3.9) | 0.195 |
| TTCW (mnths) | 5.4 (0.6–29.2) | 36.5 (7.4–63.4) | 0.032 * |
| Transplantation list (%) | 43% | 33% | 0.719 |
| Death | 40% | 25% | 0.443 |
| FU (yrs) | 3.0 (0.4–3.3) | 3.6 (3.0–5.3) | 0.687 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smits, J.; Tasev, D.; Andersen, S.; Szulcek, R.; Botros, L.; Ringgaard, S.; Andersen, A.; Vonk-Noordegraaf, A.; Koolwijk, P.; Bogaard, H.J. Blood Outgrowth and Proliferation of Endothelial Colony Forming Cells are Related to Markers of Disease Severity in Patients with Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2018, 19, 3763. https://doi.org/10.3390/ijms19123763
Smits J, Tasev D, Andersen S, Szulcek R, Botros L, Ringgaard S, Andersen A, Vonk-Noordegraaf A, Koolwijk P, Bogaard HJ. Blood Outgrowth and Proliferation of Endothelial Colony Forming Cells are Related to Markers of Disease Severity in Patients with Pulmonary Arterial Hypertension. International Journal of Molecular Sciences. 2018; 19(12):3763. https://doi.org/10.3390/ijms19123763
Chicago/Turabian StyleSmits, Josien, Dimitar Tasev, Stine Andersen, Robert Szulcek, Liza Botros, Steffen Ringgaard, Asger Andersen, Anton Vonk-Noordegraaf, Pieter Koolwijk, and Harm Jan Bogaard. 2018. "Blood Outgrowth and Proliferation of Endothelial Colony Forming Cells are Related to Markers of Disease Severity in Patients with Pulmonary Arterial Hypertension" International Journal of Molecular Sciences 19, no. 12: 3763. https://doi.org/10.3390/ijms19123763
APA StyleSmits, J., Tasev, D., Andersen, S., Szulcek, R., Botros, L., Ringgaard, S., Andersen, A., Vonk-Noordegraaf, A., Koolwijk, P., & Bogaard, H. J. (2018). Blood Outgrowth and Proliferation of Endothelial Colony Forming Cells are Related to Markers of Disease Severity in Patients with Pulmonary Arterial Hypertension. International Journal of Molecular Sciences, 19(12), 3763. https://doi.org/10.3390/ijms19123763