Interactive Responses of Solanum Dulcamara to Drought and Insect Feeding are Herbivore Species-Specific
Abstract
:1. Introduction
2. Results
2.1. Drought Treatment Differentially Affects Herbivore Performance
2.2. Water Availability Interacts with Herbivory in Regulating Plant Hormone Levels
2.3. Transcriptional Regulation
2.4. Drought and Insect Herbivory Increase Serine-Type PI (serPI) Levels and PI Gene Expression
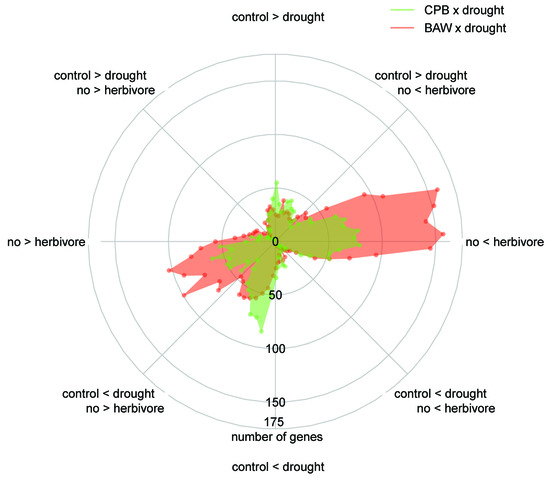
2.5. BAW Elicits More Prominent Responses than CPB, Especially under Drought
2.6. Drought Enhances Specific Responses to Each Herbivore
2.7. Herbivore Responses that Were not Induced by CPB in Drought-Stressed Plants
2.8. Plant Responses to Drought Are More Prominent under CPB Herbivory
3. Discussion
3.1. Drought Enhances Defence Responses to BAW
3.2. Interaction between Drought and CPB Herbivory Responses Benefits the Herbivore
3.3. The PI Response is Enhanced by Drought, but Does Not Decrease CPB Performance
4. Materials and Methods
4.1. Plant Materials
4.2. Watering Treatments
4.3. Herbivory Treatments and Insect Performance
4.4. Quantification of PI and Total Protein Contents
4.5. Hormone Quantification
4.6. Microarray Analysis
4.7. Quantitative PCR (qPCR) Validation of Microarray Data
4.8. Gene Set Enrichment Analysis and Clustering of Transcriptomic Response
4.9. On-Line Detection of ET Emission
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Herms, D.A.; Mattson, W.J. The dilemma of plants: To grow or defend. Q. Rev. Biol. 1992, 67, 283–335. [Google Scholar] [CrossRef]
- Skirycz, A.; Inzé, D. More from less: Plant growth under limited water. Curr. Opin. Biotechnol. 2010, 21, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Vos, I.A.; Pieterse, C.M.J.; van Wees, S.C.M. Costs and benefits of hormone-regulated plant defences. Plant Pathol. 2013, 62, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef] [PubMed]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth-defense tradeoffs in plants: A balancing act to optimize fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Rieu, I.; Mariani, C.; van Dam, N.M. How plants handle multiple stresses: Hormonal interactions underlying responses to abiotic stress and insect herbivory. Plant Mol. Biol. 2016, 91, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Meldau, S.; Howe, G.A. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012, 17, 250–259. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.J.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C.M. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef]
- Acevedo, F.E.; Rivera-Vega, L.J.; Chung, S.H.; Ray, S.; Felton, G.W. Cues from chewing insects—The intersection of DAMPs, HAMPs, MAMPs and effectors. Curr. Opin. Plant Biol. 2015, 26, 80–86. [Google Scholar] [CrossRef]
- Diezel, C.; von Dahl, C.C.; Gaquerel, E.; Baldwin, I.T. Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol. 2009, 150, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- Rehrig, E.M.; Appel, H.M.; Jones, A.D.; Schultz, J.C. Roles for jasmonate- and ethylene-induced transcription factors in the ability of Arabidopsis to respond differentially to damage caused by two insect herbivores. Front. Plant Sci. 2014, 5, 407. [Google Scholar] [CrossRef] [PubMed]
- Vos, I.A.; Verhage, A.; Schuurink, R.C.; Watt, L.G.; Pieterse, C.M.J.; Van Wees, S.C.M. Onset of herbivore-induced resistance in systemic tissue primed for jasmonate-dependent defenses is activated by abscisic acid. Front. Plant Sci. 2013, 4, 539. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, O.; Chico, J.M.; Sa1chez-Serrano, J.J.; Solano, R. JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 2004, 16, 1938–1950. [Google Scholar] [CrossRef] [PubMed]
- Dombrecht, B.; Xue, G.P.; Sprague, S.J.; Kirkegaard, J.A.; Ross, J.J.; Reid, J.B.; Fitt, G.P.; Sewelam, N.; Schenk, P.M.; Manners, J.M.; et al. MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 2007, 19, 2225–2245. [Google Scholar] [CrossRef]
- Schweizer, F.; Fernández-Calvo, P.; Zander, M.; Diez-Diaz, M.; Fonseca, S.; Glauser, G.; Lewsey, M.G.; Ecker, J.R.; Solano, R.; Reymond, P. Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 2013, 25, 3117–3132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankala, A.; Luthe, D.S.; Williams, W.P.; Wilkinson, J.R. Integration of ethylene and jasmonic acid signaling pathways in the expression of maize defense protein Mir1-CP. Mol. Plant Microbe Interact. 2009, 22, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Onkokesung, N.; Baldwin, I.T.; Gális, I. The role of jasmonic acid and ethylene crosstalk in direct defense of Nicotiana attenuata plants against chewing herbivores. Plant Signal. Behav. 2010, 5, 1305–1307. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.P.; Badruzsaufari, E.; Schenk, P.M.; Manners, J.M.; Desmond, O.J.; Ehlert, C.; Maclean, D.J.; Ebert, P.R.; Kazan, K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 2004, 16, 3460–3479. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Huang, H.; Gao, H.; Wang, J.; Wu, D.; Liu, X.; Yang, S.; Zhai, Q.; Li, C.; Qi, T.; et al. Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between jasmonate and ethylene signaling in Arabidopsis. Plant Cell 2014, 26, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Tomitaka, Y.; Shimoda, T.; Seo, S.; Sakurai, T.; Kugimiya, S.; Tsuda, S.; Kobayashi, M. Antagonistic plant defense system regulated by phytohormones assists interactions among vector insect, thrips and a tospovirus. Plant Cell Physiol. 2012, 53, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Rosa, C.; Scully, E.D.; Peiffer, M.; Tooker, J.F.; Hoover, K.; Luthe, D.S.; Felton, G.W. Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc. Natl. Acad. Sci. USA 2013, 110, 15728–15733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danner, H.; Desurmont, G.A.; Cristescu, S.M.; van Dam, N.M. Herbivore-induced plant volatiles accurately predict history of coexistence, diet breadth, and feeding mode of herbivores. New Phytol. 2018, 220, 726–738. [Google Scholar] [CrossRef] [PubMed]
- Drok, S.; Bandoly, M.; Stelzer, S.; Lortzing, T.; Steppuhn, A. Moth oviposition shapes the species-specific transcriptional and phytohormonal response of Nicotiana attenuata to larval feeding. Sci. Rep. 2018, 8, 10249. [Google Scholar] [CrossRef] [PubMed]
- Voelckel, C.; Baldwin, I.T. Generalist and specialist lepidopteran larvae elicit different transcriptional responses in Nicotiana attenuata, which correlate with larval FAC profiles. Ecol. Lett. 2004, 7, 770–775. [Google Scholar] [CrossRef]
- Ali, J.G.; Agrawal, A.A. Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci. 2012, 17, 293–302. [Google Scholar] [CrossRef]
- Sharp, R.E.; Poroyko, V.; Hejlek, L.G.; Spollen, W.G.; Springer, G.K.; Bohnert, H.J.; Nguyen, H.T. Root growth maintenance during water deficits: Physiology to functional genomics. J. Exp. Bot. 2004, 55, 2343–2351. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Szarejko, I. Open or close the gate—Stomata action under the control of phytohormones in drought stress conditions. Front. Plant Sci. 2013, 4, 138. [Google Scholar] [CrossRef]
- Liang, C.; Wang, Y.; Zhu, Y.; Tang, J.; Hu, B.; Liu, L.; Ou, S.; Wu, H.; Sun, X.; Chu, J.; et al. OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proc. Natl. Acad. Sci. USA 2014, 111, 10013–10018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Chang, C.; Tucker, M.L. To grow old: Regulatory role of ethylene and jasmonic acid in senescence. Front. Plant Sci. 2015, 6, 20. [Google Scholar] [CrossRef]
- Hossain, M.A.; Munemasa, S.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Involvement of endogenous abscisic acid in methyl jasmonate-induced stomatal closure in Arabidopsis. Plant Physiol. 2011, 156, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Munemasa, S.; Hossain, M.A.; Nakamura, Y.; Mori, I.C.; Murata, Y. The Arabidopsis calcium-dependent protein kinase, CPK6, functions as a positive regulator of methyl jasmonate signaling in guard cells. Plant Physiol. 2011, 155, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Okamoto, H.; Okuma, E.; Shiba, H.; Kamada, H.; Hasegawa, P.M.; Murata, Y. SIZ1 deficiency causes reduced stomatal aperture and enhanced drought tolerance via controlling salicylic acid-induced accumulation of reactive oxygen species in Arabidopsis. Plant J. 2013, 73, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Sharp, R.E.; LeNoble, M.E. ABA, ethylene and the control of shoot and root growth under water stress. J. Exp. Bot. 2002, 53, 33–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Sano, T.; Tamaoki, M.; Nakajima, N.; Kondo, N.; Hasezawa, S. Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol. 2005, 138, 2337–2343. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.-C.; Ma, B.; Collinge, D.P.; Pogson, B.J.; He, S.-J.; Xiong, Q.; Duan, K.-X.; Chen, H.; Yang, C.; Lu, X.; et al. Ethylene responses in rice roots and coleoptiles are differentially regulated by a carotenoid isomerase-mediated abscisic acid pathway. Plant Cell 2015, 27, 1061–1081. [Google Scholar] [CrossRef] [PubMed]
- Gutbrodt, B.; Mody, K.; Dorn, S. Drought changes plant chemistry and causes contrasting responses in lepidopteran herbivores. Oikos 2011, 120, 1732–1740. [Google Scholar] [CrossRef]
- Weldegergis, B.T.; Zhu, F.; Poelman, E.H.; Dicke, M. Drought stress affects plant metabolites and herbivore preference but not host location by its parasitoids. Oecologia 2015, 177, 701–713. [Google Scholar] [CrossRef]
- Stone, C.; Chesnut, K.; Penman, T.; Nichols, D. Waterlogging increases the infestation level of the pest psyllid Creiis lituratus on Eucalyptus dunnii. Aust. For. 2010, 73, 98–105. [Google Scholar] [CrossRef]
- Simpson, K.L.S.; Jackson, G.E.; Grace, J. The response of aphids to plant water stress—the case of Myzus persicae and Brassica oleracea var. capitata. Entomol. Exp. Appl. 2012, 142, 191–202. [Google Scholar] [CrossRef]
- Zhang, Q.; Visser, E.J.W.; de Kroon, H.; Huber, H. Life cycle stage and water depth affect flooding-induced adventitious root formation in the terrestrial species Solanum dulcamara. Ann. Bot. 2015, 116, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Calf, O.; van Dam, N.M. Bittersweet bugs: The Dutch insect community on the nightshade Solanum dulcamara. Entomol. Ber. 2012, 72, 193–198. [Google Scholar]
- Nguyen, D.; D’Agostino, N.; Tytgat, T.O.G.; Sun, P.; Lortzing, T.; Visser, E.J.W.; Cristescu, S.M.; Steppuhn, A.; Mariani, C.; van Dam, N.M.; et al. Drought and flooding have distinct effects on herbivore-induced responses and resistance in Solanum dulcamara. Plant Cell Environ. 2016, 39, 1485–1499. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Caarls, L.; Pieterse, C.M.J.; Van Wees, S.C.M. How salicylic acid takes transcriptional control over jasmonic acid signaling. Front. Plant Sci. 2015, 6, 170. [Google Scholar] [CrossRef] [PubMed]
- Malinovsky, F.G.; Fangel, J.U.; Willats, W.G.T. The role of the cell wall in plant immunity. Front. Plant Sci. 2014, 5, 178. [Google Scholar] [CrossRef] [PubMed]
- Ferrieri, A.P.; Arce, C.C.M.; Machado, R.A.R.; Meza-Canales, I.D.; Lima, E.; Baldwin, I.T.; Erb, M. A Nicotiana attenuata cell wall invertase inhibitor (NaCWII) reduces growth and increases secondary metabolite biosynthesis in herbivore-attacked plants. New Phytol. 2015, 208, 519–530. [Google Scholar] [CrossRef]
- Santiago, R.; Barros-Rios, J.; Malvar, R.A. Impact of cell wall composition on maize resistance to pests and diseases. Int. J. Mol. Sci. 2013, 14, 6960–6980. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, S.D.; Novak, N.G.; Ju, C.J.-T.; Cooke, J.E.K. Potato, Solanum tuberosum, defense against Colorado potato beetle, Leptinotarsa decemlineata (Say): Microarray gene expression profiling of potato by Colorado potato beetle regurgitant treatment of wounded leaves. J. Chem. Ecol. 2008, 34, 1013–1025. [Google Scholar] [CrossRef]
- Chung, S.H.; Felton, G.W. Specificity of induced resistance in tomato against specialist lepidopteran and coleopteran species. J. Chem. Ecol. 2011, 37, 378–386. [Google Scholar] [CrossRef]
- Muratoglu, H.; Demirbag, Z.; Sezen, K. The first investigation of the diversity of bacteria associated with Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Biologia (Bratisl) 2011, 66, 288–293. [Google Scholar] [CrossRef]
- Kumar, A.; Congiu, L.; Lindström, L.; Piiroinen, S.; Vidotto, M.; Grapputo, A. Sequencing, De Novo assembly and annotation of the Colorado Potato Beetle, Leptinotarsa decemlineata, transcriptome. PLoS ONE 2014, 9, e86012. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.-K.; Wang, C.Y. The dual effects of methyl salicylate on ripening and expression of ethylene biosynthetic genes in tomato fruit. Plant Sci. 2003, 164, 589–596. [Google Scholar] [CrossRef]
- Vahala, J.; Keinänen, M.; Schützendübel, A.; Polle, A.; Kangasjärvi, J. Differential effects of elevated ozone on two hybrid aspen genotypes predisposed to chronic ozone fumigation. Role of ethylene and salicylic acid. Plant Physiol. 2003, 132, 196–205. [Google Scholar] [CrossRef] [PubMed]
- LeNoble, M.E.; Spollen, W.G.; Sharp, R.E. Maintenance of shoot growth by endogenous ABA: Genetic assessment of the involvement of ethylene suppression. J. Exp. Bot. 2004, 55, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Huberty, A.F.; Denno, R.F. Plant water stress and its consequences for herbivorous insects: A new synthesis. Ecology 2004, 85, 1383–1398. [Google Scholar] [CrossRef]
- Simova-Stoilova, L.; Vaseva, I.; Grigorova, B.; Demirevska, K.; Feller, U. Proteolytic activity and cysteine protease expression in wheat leaves under severe soil drought and recovery. Plant Physiol. Biochem. 2010, 48, 200–206. [Google Scholar] [CrossRef]
- Rivas-Ubach, A.; Gargallo-Garriga, A.; Sardans, J.; Oravec, M.; Mateu-Castell, L.; Pérez-Trujillo, M.; Parella, T.; Ogaya, R.; Urban, O.; Peñuelas, J. Drought enhances folivory by shifting foliar metabolomes in Quercus ilex trees. New Phytol. 2014, 202, 874–885. [Google Scholar] [CrossRef]
- Challabathula, D.; Puthur, J.T.; Bartels, D. Surviving metabolic arrest: Photosynthesis during desiccation and rehydration in resurrection plants. Ann. N. Y. Acad. Sci. 2015. [Google Scholar] [CrossRef]
- Couture, J.J.; Serbin, S.P.; Townsend, P.A. Elevated temperature and periodic water stress alter growth and quality of common milkweed (Asclepias syriaca) and monarch (Danaus plexippus) larval performance. Arthropod Plant Interact. 2015, 9, 149–161. [Google Scholar] [CrossRef]
- Desclos, M.; Dubousset, L.; Etienne, P.; Le Caherec, F.; Satoh, H.; Bonnefoy, J.; Ourry, A.; Avice, J.-C. A proteomic profiling approach to reveal a novel role of Brassica napus drought 22 kD/water-soluble chlorophyll-binding protein in young leaves during nitrogen remobilization induced by stressful conditions. Plant Physiol. 2008, 147, 1830–1844. [Google Scholar] [CrossRef] [PubMed]
- Mosolov, V.V.; Valueva, T.A. Inhibitors of proteolytic enzymes under abiotic stresses in plants (review). Appl. Biochem. Microbiol. 2011, 47, 453–459. [Google Scholar] [CrossRef]
- Martinez-Medina, A.; Flors, V.; Heil, M.; Mauch-Mani, B.; Pieterse, C.M.J.; Pozo, M.J.; Ton, J.; van Dam, N.M.; Conrath, U. Recognizing plant defense priming. Trends Plant Sci. 2016, 21, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Peña-Cortés, H.; Fisahn, J.; Willmitzer, L. Signals involved in wound-induced proteinase inhibitor II gene expression in tomato and potato plants. Proc. Natl. Acad. Sci. USA 1995, 92, 4106–4113. [Google Scholar] [CrossRef] [PubMed]
- Carrera, E.; Prat, S. Expression of the Arabidopsis abi1-1 mutant allele inhibits proteinase inhibitor wound-induction in tomato. Plant J. 1998, 15, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Zhu-Salzman, K.; Zeng, R. Insect response to plant defensive protease inhibitors. Annu. Rev. Entomol. 2015, 60, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Bolter, C.J.; Jongsma, M.A. Colorado potato beetles (Leptinotarsa decemlineata) adapt to proteinase inhibitors induced in potato leaves by methyl jasmonate. J. Insect Physiol. 1995, 41, 1071–1078. [Google Scholar] [CrossRef]
- Jongsma, M.A.; Bakker, P.L.; Peters, J.; Bosch, D.; Stiekema, W.J. Adaptation of Spodoptera exigua larvae to plant proteinase inhibitors by induction of gut proteinase activity insensitive to inhibition. Proc. Natl. Acad. Sci. USA 1995, 92, 8041–8045. [Google Scholar] [CrossRef]
- Lara, P.; Ortego, F.; Gonzalez-Hidalgo, E.; Castañera, P.; Carbonero, P.; Diaz, I. Adaptation of Spodoptera exigua (Lepidoptera: Noctuidae) to barley trypsin inhibitor BTI-CMe expressed in transgenic tobacco. Transgenic Res. 2000, 9, 169–178. [Google Scholar] [CrossRef]
- Brunelle, F.; Cloutier, C.; Michaud, D. Colorado potato beetles compensate for tomato cathepsin D inhibitor expressed in transgenic potato. Arch. Insect Biochem. Physiol. 2004, 55, 103–113. [Google Scholar] [CrossRef]
- Govind, G.; Mittapalli, O.; Griebel, T.; Allmann, S.; Böcker, S.; Baldwin, I.T. Unbiased transcriptional comparisons of generalist and specialist herbivores feeding on progressively defenseless Nicotiana attenuata plants. PLoS ONE 2010, 5, e8735. [Google Scholar] [CrossRef] [PubMed]
- Petek, M.; Turnšek, N.; Gašparič, M.B.; Novak, M.P.; Gruden, K.; Slapar, N.; Popovič, T.; Štrukelj, B.; Jongsma, M.A. A complex of genes involved in adaptation of Leptinotarsa decemlineata larvae to induced potato defense. Arch. Insect Biochem. Physiol. 2012, 79, 153–181. [Google Scholar] [CrossRef] [PubMed]
- Ortego, F.; Novillo, C.; Sánchez-Serrano, J.J.; Castañera, P. Physiological response of Colorado potato beetle and beet armyworm larvae to depletion of wound-inducible proteinase inhibitors in transgenic potato plants. J. Insect Physiol. 2001, 47, 1291–1300. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Lawson, F.R.; Yamamoto, R.; Smith, C.N. Insect Colonization and Mass Production; Academic Press: New York, NY, USA, 1966. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Method 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Stout, M.J.; Karban, R.; Duffey, S.S. Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J. Chem. Ecol. 1996, 22, 1767–1781. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bode, R.F.; Halitschke, R.; Kessler, A. Herbivore damage-induced production and specific anti-digestive function of serine and cysteine protease inhibitors in tall goldenrod, Solidago altissima L. (Asteraceae). Planta 2013, 237, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Halitschke, R.; Kang, J.-H.; Berg, A.; Harnisch, F.; Baldwin, I.T. Independently silencing two JAR family members impairs levels of trypsin proteinase inhibitors but not nicotine. Planta 2007, 226, 159–167. [Google Scholar] [CrossRef]
- D’Agostino, N.; Golas, T.; van de Geest, H.; Bombarely, A.; Dawood, T.; Zethof, J.; Driedonks, N.; Wijnker, E.; Bargsten, J.; Nap, J.-P.; et al. Genomic analysis of the native European Solanum species, S. dulcamara. BMC Genom. 2013, 14, 356. [Google Scholar] [CrossRef] [Green Version]
- Lortzing, T.; Firtzlaff, V.; Nguyen, D.; Rieu, I.; Stelzer, S.; Schad, M.; Kallarackal, J.; Steppuhn, A. Transcriptomic responses of Solanum dulcamara to natural and simulated herbivory. Mol. Ecol. Resour. 2017, 17, e196–e211. [Google Scholar] [CrossRef]
- Rieu, I.; Powers, S.J. Real-time quantitative RT-PCR: Design, calculations, and statistics. Plant Cell 2009, 21, 1031–1033. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]









| Group of Biological Processes | Upregulated | Downregulated | Total |
|---|---|---|---|
| Amino acid metabolism | 16 | 0 | 16 |
| Carbohydrate metabolism | 14 | 0 | 14 |
| Cell wall remodelling | 19 | 0 | 19 |
| Developmental processes | 4 | 0 | 4 |
| Hormonal homeostasis and signalling | 14 | 0 | 14 |
| Ion homeostasis | 7 | 0 | 7 |
| Lipid metabolism | 11 | 0 | 11 |
| Photosynthesis-related processes | 1 | 7 | 8 |
| Redox homeostasis | 8 | 3 | 11 |
| Responses to abiotic stresses | 6 | 4 | 10 |
| Responses to biotic stresses | 15 | 0 | 15 |
| Secondary metabolism | 20 | 0 | 20 |
| Other molecular physiological processes | 10 | 6 | 16 |
| Total | 145 | 20 | 165 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.; Poeschl, Y.; Lortzing, T.; Hoogveld, R.; Gogol-Döring, A.; Cristescu, S.M.; Steppuhn, A.; Mariani, C.; Rieu, I.; Van Dam, N.M. Interactive Responses of Solanum Dulcamara to Drought and Insect Feeding are Herbivore Species-Specific. Int. J. Mol. Sci. 2018, 19, 3845. https://doi.org/10.3390/ijms19123845
Nguyen D, Poeschl Y, Lortzing T, Hoogveld R, Gogol-Döring A, Cristescu SM, Steppuhn A, Mariani C, Rieu I, Van Dam NM. Interactive Responses of Solanum Dulcamara to Drought and Insect Feeding are Herbivore Species-Specific. International Journal of Molecular Sciences. 2018; 19(12):3845. https://doi.org/10.3390/ijms19123845
Chicago/Turabian StyleNguyen, Duy, Yvonne Poeschl, Tobias Lortzing, Rick Hoogveld, Andreas Gogol-Döring, Simona M. Cristescu, Anke Steppuhn, Celestina Mariani, Ivo Rieu, and Nicole M. Van Dam. 2018. "Interactive Responses of Solanum Dulcamara to Drought and Insect Feeding are Herbivore Species-Specific" International Journal of Molecular Sciences 19, no. 12: 3845. https://doi.org/10.3390/ijms19123845
APA StyleNguyen, D., Poeschl, Y., Lortzing, T., Hoogveld, R., Gogol-Döring, A., Cristescu, S. M., Steppuhn, A., Mariani, C., Rieu, I., & Van Dam, N. M. (2018). Interactive Responses of Solanum Dulcamara to Drought and Insect Feeding are Herbivore Species-Specific. International Journal of Molecular Sciences, 19(12), 3845. https://doi.org/10.3390/ijms19123845