Circadian Rhythm Abnormalities in Parkinson’s Disease from Humans to Flies and Back
Abstract
:1. Introduction
2. Circadian Abnormalities in PD Pathology
2.1. Circadian Symptoms in PD Patients
2.2. Circadian Dysfunctions in Mammalian PD Models
3. Drosophila melanogaster as a Model Organism to Study the Relationship between the Circadian Clock and PD
3.1. The Drosophila Circadian Clock
3.2. Drosophila Dopaminergic System
3.3. Drosophila PD Models and Circadian Dysfunctions
3.3.1. α-syn
3.3.2. Parkin and PINK1
3.3.3. DJ-1
4. Drosophila as a Model to Evaluate the Effect of Circadian Disruptions on Neurodegenerative Processes
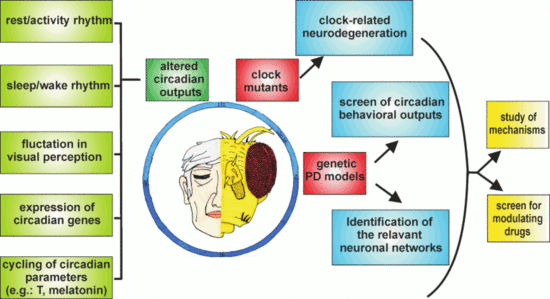
5. Conclusions and Further Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shults, C.W. Lewy bodies. Proc. Natl. Acad. Sci. USA 2006, 103, 1661–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barone, P.; Antonini, A.; Colosimo, C.; Marconi, R.; Morgante, L.; Avarello, T.P.; Bottacchi, E.; Cannas, A.; Ceravolo, G.; Ceravolo, R.; et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov. Disord. 2009, 24, 1641–1649. [Google Scholar] [CrossRef]
- Videnovic, A.; Golombek, D. Circadian Dysregulation in Parkinson’s Disease. Neurobiol. Sleep Circadian Rhythm. 2017, 2, 53–58. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Wang, F.; Hu, L.-F.; Liu, C.-F. A New Perspective for Parkinson’s Disease: Circadian Rhythm. Neurosci. Bull. 2017, 33, 62–72. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. Neuropathological Staging of Brain Pathology in Sporadic Parkinson’s disease: Separating the Wheat from the Chaff. J. Parkinson’s Dis. 2017, 7, S71–S85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boeve, B.F. REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann. N. Y. Acad. Sci. 2010, 1184, 15–54. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, K.S.; Blakemore, L.J.; Trombley, P.Q. Dopamine: A Modulator of Circadian Rhythms in the Central Nervous System. Front. Cell. Neurosci. 2017, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Moghadam, A.; Charsouei, S.; Bell, B.; Jabalameli, M.R. Parkinson Disease from Mendelian Forms to Genetic Susceptibility: New Molecular Insights into the Neurodegeneration Process. Cell. Mol. Neurobiol. 2018, 38, 1153–1178. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, U. Timing to perfection: The biology of central and peripheral circadian clocks. Neuron 2012, 74, 246–260. [Google Scholar] [CrossRef]
- Piccini, P.; Del Dotto, P.; Pardini, C.; D’Antonio, P.; Rossi, G.; Bonuccelli, U. Diurnal worsening in Parkinson patients treated with levodopa. Riv. Neurol. 1991, 61, 219–224. [Google Scholar]
- Van Hilten, J.J.; Hoogland, G.; van der Velde, E.A.; Middelkoop, H.A.; Kerkhof, G.A.; Roos, R.A. Diurnal effects of motor activity and fatigue in Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1993, 56, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Placidi, F.; Izzi, F.; Romigi, A.; Stanzione, P.; Marciani, M.G.; Brusa, L.; Sperli, F.; Galati, S.; Pasqualetti, P.; Pierantozzi, M. Sleep-wake cycle and effects of cabergoline monotherapy in de novo Parkinson’s disease patients. An ambulatory polysomnographic study. J. Neurol. 2008, 255, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Prudon, B.; Duncan, G.W.; Khoo, T.K.; Yarnall, A.J.; Anderson, K.N. Primary sleep disorder prevalence in patients with newly diagnosed Parkinson’s disease. Mov. Disord. 2014, 29, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Prieto-Jurcynska, C.; Naidu, Y.; Mitra, T.; Frades-Payo, B.; Tluk, S.; Ruessmann, A.; Odin, P.; Macphee, G.; Stocchi, F.; et al. The nondeclaration of nonmotor symptoms of Parkinson’s disease to health care professionals: An international study using the nonmotor symptoms questionnaire. Mov. Disord. 2010, 25, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Avidan, A.; Hays, R.D.; Diaz, N.; Bordelon, Y.; Thompson, A.W.; Vassar, S.D.; Vickrey, B.G. Associations of sleep disturbance symptoms with health-related quality of life in Parkinson’s disease. J. Neuropsychiatry Clin. Neurosci. 2013, 25, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Weil, R.S.; Schrag, A.E.; Warren, J.D.; Crutch, S.J.; Lees, A.J.; Morris, H.R. Visual dysfunction in Parkinson’s disease. Brain 2016, 139, 2827–2843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulens, C.; Meerwaldt, J.D.; Van der Wildt, G.J.; Van Deursen, J.B. Effect of levodopa treatment on contrast sensitivity in Parkinson’s disease. Ann. Neurol. 1987, 22, 365–369. [Google Scholar] [CrossRef]
- Büttner, T.; Kuhn, W.; Patzold, T.; Przuntek, H. L-Dopa improves colour vision in Parkinson’s disease. J. Neural Transm. Park Dis. Dement. Sect. 1994, 7, 13–19. [Google Scholar] [CrossRef]
- Struck, L.K.; Rodnitzky, R.L.; Dobson, J.K. Circadian fluctuations of contrast sensitivity in Parkinson’s disease. Neurology 1990, 40, 467–470. [Google Scholar] [CrossRef]
- Zhong, G.; Bolitho, S.; Grunstein, R.; Naismith, S.L.; Lewis, S.J.G. The relationship between thermoregulation and REM sleep behaviour disorder in Parkinson’s disease. PLoS ONE 2013, 8, e72661. [Google Scholar] [CrossRef]
- Ejaz, A.A.; Sekhon, I.S.; Munjal, S. Characteristic findings on 24-h ambulatory blood pressure monitoring in a series of patients with Parkinson’s disease. Eur. J. Intern. Med. 2006, 17, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T.; Kitano, Y.; Kuno, S. Blood pressure fluctuation and hypertension in patients with Parkinson’s disease. Brain Behav. 2013, 3, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Breen, D.P.; Vuono, R.; Nawarathna, U.; Fisher, K.; Shneerson, J.M.; Reddy, A.B.; Barker, R.A. Sleep and circadian rhythm regulation in early Parkinson disease. JAMA Neurol. 2014, 71, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Videnovic, A.; Noble, C.; Reid, K.J.; Peng, J.; Turek, F.W.; Marconi, A.; Rademaker, A.W.; Simuni, T.; Zadikoff, C.; Zee, P.C. Circadian melatonin rhythm and excessive daytime sleepiness in Parkinson disease. JAMA Neurol. 2014, 71, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Bolitho, S.J.; Naismith, S.L.; Rajaratnam, S.M.W.; Grunstein, R.R.; Hodges, J.R.; Terpening, Z.; Rogers, N.; Lewis, S.J.G. Disturbances in melatonin secretion and circadian sleep-wake regulation in Parkinson disease. Sleep Med. 2014, 15, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Liu, S.; Sothern, R.B.; Xu, S.; Chan, P. Expression of clock genes Per1 and Bmal1 in total leukocytes in health and Parkinson’s disease. Eur. J. Neurol. 2010, 17, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Wang, B.; Zhang, Y.-B.; Ding, H.; Zhang, Y.; Yu, J.; Gu, M.; Chan, P.; Cai, Y. Association of ARNTL and PER1 genes with Parkinson’s disease: A case-control study of Han Chinese. Sci. Rep. 2015, 5, 15891. [Google Scholar] [CrossRef] [PubMed]
- Schnell, A.; Albrecht, U.; Sandrelli, F. Rhythm and mood: Relationships between the circadian clock and mood-related behavior. Behav. Neurosci. 2014, 128, 326–343. [Google Scholar] [CrossRef] [PubMed]
- Thenganatt, M.A.; Jankovic, J. Parkinson disease subtypes. JAMA Neurol. 2014, 71, 499–504. [Google Scholar] [CrossRef]
- Fifel, K. Alterations of the circadian system in Parkinson’s disease patients. Mov. Disord. 2017, 32, 682–692. [Google Scholar] [CrossRef]
- Kudo, T.; Loh, D.H.; Truong, D.; Wu, Y.; Colwell, C.S. Circadian dysfunction in a mouse model of Parkinson’s disease. Exp. Neurol. 2011, 232, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Fifel, K.; Cooper, H.M. Loss of dopamine disrupts circadian rhythms in a mouse model of Parkinson’s disease. Neurobiol. Dis. 2014, 71, 359–369. [Google Scholar] [CrossRef]
- Fifel, K.; Dkhissi-Benyahya, O.; Cooper, H.M. Lack of long-term changes in circadian, locomotor, and cognitive functions in acute and chronic MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse models of Parkinson’s disease. Chronobiol. Int. 2013, 30, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Yamaguchi, E.; Takahashi, M.; Hashimura, K.; Shibata, T.; Nakamura, W.; Nakamura, T.J. Effects of age-related dopaminergic neuron loss in the substantia nigra on the circadian rhythms of locomotor activity in mice. Neurosci. Res. 2012, 74, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, A.; Matsunaga, N.; Okazaki, H.; Kakimoto, K.; Kimura, Y.; Azuma, H.; Ikeda, E.; Shiba, T.; Yamato, M.; Yamada, K.-I.; et al. A disruption mechanism of the molecular clock in a MPTP mouse model of Parkinson’s disease. Neuromol. Med. 2013, 15, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Hineno, T.; Mizobuchi, M.; Hiratani, K.; Inami, Y.; Kakimoto, Y. Disappearance of circadian rhythms in Parkinson’s disease model induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in dogs. Brain Res. 1992, 580, 92–99. [Google Scholar] [CrossRef]
- Fifel, K.; Vezoli, J.; Dzahini, K.; Claustrat, B.; Leviel, V.; Kennedy, H.; Procyk, E.; Dkhissi-Benyahya, O.; Gronfier, C.; Cooper, H.M. Alteration of daily and circadian rhythms following dopamine depletion in MPTP treated non-human primates. PLoS ONE 2014, 9, e86240. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, D.; Liu, W.; Li, S.; Chen, J.; Shen, Y.; Wang, F.; Hu, L.-F.; Liu, C.-F. Disruption of the Circadian Clock Alters Antioxidative Defense via the SIRT1-BMAL1 Pathway in 6-OHDA-Induced Models of Parkinson’s Disease. Oxid. Med. Cell. Longev. 2018, 2018, 4854732. [Google Scholar] [CrossRef]
- Gravotta, L.; Gavrila, A.M.; Hood, S.; Amir, S. Global depletion of dopamine using intracerebroventricular 6-hydroxydopamine injection disrupts normal circadian wheel-running patterns and PERIOD2 expression in the rat forebrain. J. Mol. Neurosci. 2011, 45, 162–171. [Google Scholar] [CrossRef]
- Isobe, Y.; Nishino, H. Circadian rhythm of drinking and running-wheel activity in rats with 6-hydroxydopamine lesions of the ventral tegmental area. Brain Res. 2001, 899, 187–192. [Google Scholar] [CrossRef]
- Ben, V.; Bruguerolle, B. Effects of bilateral striatal 6-OHDA lesions on circadian rhythms in the rat: A radiotelemetric study. Life Sci. 2000, 67, 1549–1558. [Google Scholar] [CrossRef]
- Li, S.-Y.; Wang, Y.-L.; Liu, W.-W.; Lyu, D.-J.; Wang, F.; Mao, C.-J.; Yang, Y.-P.; Hu, L.-F.; Liu, C.-F. Long-term Levodopa Treatment Accelerates the Circadian Rhythm Dysfunction in a 6-hydroxydopamine Rat Model of Parkinson’s Disease. Chin. Med. J. 2017, 130, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Glinka, Y.; Gassen, M.; Youdim, M.B. Mechanism of 6-hydroxydopamine neurotoxicity. J. Neural Transm. Suppl. 1997, 50, 55–66. [Google Scholar] [PubMed]
- Burns, R.S.; Chiueh, C.C.; Markey, S.P.; Ebert, M.H.; Jacobowitz, D.M.; Kopin, I.J. A primate model of parkinsonism: Selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc. Natl. Acad. Sci. USA 1983, 80, 4546–4550. [Google Scholar] [CrossRef] [PubMed]
- Lauretti, E.; Di Meco, A.; Merali, S.; Praticò, D. Circadian rhythm dysfunction: A novel environmental risk factor for Parkinson’s disease. Mol. Psychiatry 2017, 22, 280–286. [Google Scholar] [CrossRef]
- Mendoza, J.; Challet, E. Circadian insights into dopamine mechanisms. Neuroscience 2014, 282, 230–242. [Google Scholar] [CrossRef]
- Witkovsky, P. Dopamine and retinal function. Doc. Ophthalmol. 2004, 108, 17–40. [Google Scholar] [CrossRef]
- Bellen, H.J.; Tong, C.; Tsuda, H. 100 years of Drosophila research and its impact on vertebrate neuroscience: A history lesson for the future. Nat. Rev. Neurosci. 2010, 11, 514–522. [Google Scholar] [CrossRef]
- Hales, K.G.; Korey, C.A.; Larracuente, A.M.; Roberts, D.M. Genetics on the Fly: A Primer on the Drosophila Model System. Genetics 2015, 201, 815–842. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Soriano, V.; Paricio, N. Drosophila models of Parkinson’s disease: Discovering relevant pathways and novel therapeutic strategies. Parkinson’s Dis. 2011, 2011, 520640. [Google Scholar] [CrossRef]
- Xiong, Y.; Yu, J. Modeling Parkinson’s Disease in Drosophila: What Have We Learned for Dominant Traits? Front. Neurol. 2018, 9, 228. [Google Scholar] [CrossRef]
- Nagoshi, E. Drosophila Models of Sporadic Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 3343. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.L.; Frenkel, L.; Ceriani, M.F. The Underlying Genetics of Drosophila Circadian Behaviors. Physiology 2018, 33, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Zordan, M.A.; Sandrelli, F. Circadian Clock Dysfunction and Psychiatric Disease: Could Fruit Flies have a Say? Front. Neurol. 2015, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Dubowy, C.; Sehgal, A. Circadian Rhythms and Sleep in Drosophila melanogaster. Genetics 2017, 205, 1373–1397. [Google Scholar] [CrossRef] [Green Version]
- Allada, R.; Chung, B.Y. Circadian Organization of Behavior and Physiology in Drosophila. Annu. Rev. Physiol. 2010, 72, 605–624. [Google Scholar] [CrossRef] [PubMed]
- Peschel, N.; Helfrich-Förster, C. Setting the clock--by nature: Circadian rhythm in the fruitfly Drosophila melanogaster. FEBS Lett. 2011, 585, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Grima, B.; Chélot, E.; Xia, R.; Rouyer, F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature 2004, 431, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Stoleru, D.; Peng, Y.; Agosto, J.; Rosbash, M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 2004, 431, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Shafer, O.T. The Drosophila circadian clock is a variably coupled network of multiple peptidergic units. Science 2014, 343, 1516–1520. [Google Scholar] [CrossRef]
- Dissel, S.; Hansen, C.N.; Özkaya, Ö.; Hemsley, M.; Kyriacou, C.P.; Rosato, E. The logic of circadian organization in Drosophila. Curr. Biol. 2014, 24, 2257–2266. [Google Scholar] [CrossRef] [PubMed]
- Ozkaya, O.; Rosato, E. The circadian clock of the fly: A neurogenetics journey through time. Adv. Genet. 2012, 77, 79–123. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Wu, B.; Price, J.L.; Zhao, Z. Circadian Rhythm Neuropeptides in Drosophila: Signals for Normal Circadian Function and Circadian Neurodegenerative Disease. Int. J. Mol. Sci. 2017, 18, 886. [Google Scholar] [CrossRef] [PubMed]
- King, A.N.; Sehgal, A. Molecular and circuit mechanisms mediating circadian clock output in the Drosophila brain. Eur. J. Neurosci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Emery, P.; So, W.V.; Kaneko, M.; Hall, J.C.; Rosbash, M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 1998, 95, 669–679. [Google Scholar] [CrossRef]
- Stanewsky, R.; Kaneko, M.; Emery, P.; Beretta, B.; Wager-Smith, K.; Kay, S.A.; Rosbash, M.; Hall, J.C. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 1998, 95, 681–692. [Google Scholar] [CrossRef]
- Lin, F.J.; Song, W.; Meyer-Bernstein, E.; Naidoo, N.; Sehgal, A. Photic signaling by cryptochrome in the Drosophila circadian system. Mol. Cell. Biol. 2001, 21, 7287–7294. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.; Zheng, X.; Sehgal, A. JETLAG resets the Drosophila circadian clock by promoting light-induced degradation of TIMELESS. Science 2006, 312, 1809–1812. [Google Scholar] [CrossRef]
- Yoshii, T.; Todo, T.; Wülbeck, C.; Stanewsky, R.; Helfrich-Förster, C. Cryptochrome is present in the compound eyes and a subset of Drosophila’s clock neurons. J. Comp. Neurol. 2008, 508, 952–966. [Google Scholar] [CrossRef]
- Yoshii, T.; Hermann, C.; Helfrich-Förster, C. Cryptochrome-positive and -negative clock neurons in Drosophila entrain differentially to light and temperature. J. Biol. Rhythm. 2010, 25, 387–398. [Google Scholar] [CrossRef]
- Benito, J.; Houl, J.H.; Roman, G.W.; Hardin, P.E. The blue-light photoreceptor CRYPTOCHROME is expressed in a subset of circadian oscillator neurons in the Drosophila CNS. J. Biol. Rhythm. 2008, 23, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Helfrich-Förster, C.; Winter, C.; Hofbauer, A.; Hall, J.C.; Stanewsky, R. The circadian clock of fruit flies is blind after elimination of all known photoreceptors. Neuron 2001, 30, 249–261. [Google Scholar] [CrossRef]
- Renn, S.C.; Park, J.H.; Rosbash, M.; Hall, J.C.; Taghert, P.H. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 1999, 99, 791–802. [Google Scholar] [CrossRef]
- He, C.; Cong, X.; Zhang, R.; Wu, D.; An, C.; Zhao, Z. Regulation of circadian locomotor rhythm by neuropeptide Y-like system in Drosophila melanogaster. Insect Mol. Biol. 2013, 22, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Johard, H.A.D.; Yoishii, T.; Dircksen, H.; Cusumano, P.; Rouyer, F.; Helfrich-Förster, C.; Nässel, D.R. Peptidergic clock neurons in Drosophila: Ion transport peptide and short neuropeptide F in subsets of dorsal and ventral lateral neurons. J. Comp. Neurol. 2009, 516, 59–73. [Google Scholar] [CrossRef] [PubMed]
- King, A.N.; Barber, A.F.; Smith, A.E.; Dreyer, A.P.; Sitaraman, D.; Nitabach, M.N.; Cavanaugh, D.J.; Sehgal, A. A Peptidergic Circuit Links the Circadian Clock to Locomotor Activity. Curr. Biol. 2017, 27, 1915.e5–1927.e5. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, D.J.; Geratowski, J.D.; Wooltorton, J.R.A.; Spaethling, J.M.; Hector, C.E.; Zheng, X.; Johnson, E.C.; Eberwine, J.H.; Sehgal, A. Identification of a circadian output circuit for rest:activity rhythms in Drosophila. Cell 2014, 157, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Cavey, M.; Collins, B.; Bertet, C.; Blau, J. Circadian rhythms in neuronal activity propagate through output circuits. Nat. Neurosci. 2016, 19, 587–595. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Ng, F.; Lebestky, T.; Grygoruk, A.; Djapri, C.; Lawal, H.O.; Zaveri, H.A.; Mehanzel, F.; Najibi, R.; Seidman, G.; et al. Dispensable, redundant, complementary, and cooperative roles of dopamine, octopamine, and serotonin in Drosophila melanogaster. Genetics 2013, 193, 159–176. [Google Scholar] [CrossRef]
- Hirsh, J.; Riemensperger, T.; Coulom, H.; Iché, M.; Coupar, J.; Birman, S. Roles of dopamine in circadian rhythmicity and extreme light sensitivity of circadian entrainment. Curr. Biol. 2010, 20, 209–214. [Google Scholar] [CrossRef]
- Hanna, M.E.; Bednářová, A.; Rakshit, K.; Chaudhuri, A.; O’Donnell, J.M.; Krishnan, N. Perturbations in dopamine synthesis lead to discrete physiological effects and impact oxidative stress response in Drosophila. J. Insect Physiol. 2015, 73, 11–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, F.S.; Tangredi, M.M.; Jackson, F.R. Glial cells physiologically modulate clock neurons and circadian behavior in a calcium-dependent manner. Curr. Biol. 2011, 21, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Jackson, F.R. Drosophila ebony activity is required in glia for the circadian regulation of locomotor activity. Neuron 2007, 55, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Davis, R.L. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: Anatomical and physiological heterogeneity. Front. Neural Circuits 2009, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Friggi-Grelin, F.; Coulom, H.; Meller, M.; Gomez, D.; Hirsh, J.; Birman, S. Targeted gene expression in Drosophila dopaminergic cells using regulatory sequences from tyrosine hydroxylase. J. Neurobiol. 2003, 54, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Kasture, A.S.; Hummel, T.; Sucic, S.; Freissmuth, M. Big Lessons from Tiny Flies: Drosophila melanogaster as a Model to Explore Dysfunction of Dopaminergic and Serotonergic Neurotransmitter Systems. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Seto, E.S. Dopamine dynamics and signaling in Drosophila: An overview of genes, drugs and behavioral paradigms. Exp. Anim. 2014, 63, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Gonzalo-Gomez, A.; Turiegano, E.; León, Y.; Molina, I.; Torroja, L.; Canal, I. Ih current is necessary to maintain normal dopamine fluctuations and sleep consolidation in Drosophila. PLoS ONE 2012, 7, e36477. [Google Scholar] [CrossRef]
- Riemensperger, T.; Isabel, G.; Coulom, H.; Neuser, K.; Seugnet, L.; Kume, K.; Iché-Torres, M.; Cassar, M.; Strauss, R.; Preat, T.; et al. Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc. Natl. Acad. Sci. USA 2011, 108, 834–839. [Google Scholar] [CrossRef]
- Cichewicz, K.; Garren, E.J.; Adiele, C.; Aso, Y.; Wang, Z.; Wu, M.; Birman, S.; Rubin, G.M.; Hirsh, J. A new brain dopamine-deficient Drosophila and its pharmacological and genetic rescue. Genes Brain Behav. 2017, 16, 394–403. [Google Scholar] [CrossRef]
- Hamasaka, Y.; Nässel, D.R. Mapping of serotonin, dopamine, and histamine in relation to different clock neurons in the brain of Drosophila. J. Comp. Neurol. 2006, 494, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Abruzzi, K.C.; Zadina, A.; Luo, W.; Wiyanto, E.; Rahman, R.; Guo, F.; Shafer, O.; Rosbash, M. RNA-seq analysis of Drosophila clock and non-clock neurons reveals neuron-specific cycling and novel candidate neuropeptides. PLoS Genet. 2017, 13, e1006613. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Haynes, P.; Pírez, N.; Harrington, K.I.; Guo, F.; Pollack, J.; Hong, P.; Griffith, L.C.; Rosbash, M. Imaging analysis of clock neurons reveals light buffers the wake-promoting effect of dopamine. Nat. Neurosci. 2011, 14, 889–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potdar, S.; Sheeba, V. Wakefulness Is Promoted during Day Time by PDFR Signalling to Dopaminergic Neurons in Drosophila melanogaster. eNeuro 2018, 5. [Google Scholar] [CrossRef]
- Helfrich-Förster, C. Sleep in Insects. Annu. Rev. Entomol. 2018, 63, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Gajula Balija, M.B.; Griesinger, C.; Herzig, A.; Zweckstetter, M.; Jäckle, H. Pre-fibrillar α-synuclein mutants cause Parkinson’s disease-like non-motor symptoms in Drosophila. PLoS ONE 2011, 6, e24701. [Google Scholar] [CrossRef] [PubMed]
- Julienne, H.; Buhl, E.; Leslie, D.S.; Hodge, J.J.L. Drosophila PINK1 and parkin loss-of-function mutants display a range of non-motor Parkinson’s disease phenotypes. Neurobiol. Dis. 2017, 104, 15–23. [Google Scholar] [CrossRef]
- Valadas, J.S.; Esposito, G.; Vandekerkhove, D.; Miskiewicz, K.; Deaulmerie, L.; Raitano, S.; Seibler, P.; Klein, C.; Verstreken, P. ER Lipid Defects in Neuropeptidergic Neurons Impair Sleep Patterns in Parkinson’s Disease. Neuron 2018, 98, 1155–1169. [Google Scholar] [CrossRef]
- Feany, M.B.; Bender, W.W. A Drosophila model of Parkinson’s disease. Nature 2000, 404, 394–398. [Google Scholar] [CrossRef]
- Karpinar, D.P.; Balija, M.B.G.; Kügler, S.; Opazo, F.; Rezaei-Ghaleh, N.; Wender, N.; Kim, H.-Y.; Taschenberger, G.; Falkenburger, B.H.; Heise, H.; et al. Pre-fibrillar alpha-synuclein variants with impaired beta-structure increase neurotoxicity in Parkinson’s disease models. EMBO J. 2009, 28, 3256–3268. [Google Scholar] [CrossRef]
- Park, J.; Lee, S.B.; Lee, S.; Kim, Y.; Song, S.; Kim, S.; Bae, E.; Kim, J.; Shong, M.; Kim, J.-M.; et al. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature 2006, 441, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, A.J.; Theodore, D.A.; Greene, J.C.; Benes, H.; Wes, P.D.; Pallanck, L.J. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2005, 102, 8024–8029. [Google Scholar] [CrossRef]
- Yang, Y.; Gehrke, S.; Haque, M.E.; Imai, Y.; Kosek, J.; Yang, L.; Beal, M.F.; Nishimura, I.; Wakamatsu, K.; Ito, S.; et al. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc. Natl. Acad. Sci. USA 2005, 102, 13670–13675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menzies, F.M.; Yenisetti, S.C.; Min, K.-T. Roles of Drosophila DJ-1 in survival of dopaminergic neurons and oxidative stress. Curr. Biol. 2005, 15, 1578–1582. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, S.Y.; Cha, G.-H.; Lee, S.B.; Kim, S.; Chung, J. Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene 2005, 361, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Song, S.; Hong, Y.K.; Choi, G.; Suh, Y.S.; Han, S.Y.; Lee, M.; Park, S.H.; Lee, J.H.; Lee, S.; et al. Drosophila DJ-1 Decreases Neural Sensitivity to Stress by Negatively Regulating Daxx-Like Protein through dFOXO. PLoS Genet. 2013, 9, e1003412. [Google Scholar] [CrossRef]
- Meulener, M.; Whitworth, A.J.; Armstrong-Gold, C.E.; Rizzu, P.; Heutink, P.; Wes, P.D.; Pallanck, L.J.; Bonini, N.M. Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson’s disease. Curr. Biol. 2005, 15, 1572–1577. [Google Scholar] [CrossRef]
- Hao, L.-Y.; Giasson, B.I.; Bonini, N.M. DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function. Proc. Natl. Acad. Sci. USA 2010, 107, 9747–9752. [Google Scholar] [CrossRef] [Green Version]
- Bengoa-Vergniory, N.; Roberts, R.F.; Wade-Martins, R.; Alegre-Abarrategui, J. Alpha-synuclein oligomers: A new hope. Acta Neuropathol. 2017, 134, 819–838. [Google Scholar] [CrossRef]
- Burré, J.; Vivona, S.; Diao, J.; Sharma, M.; Brunger, A.T.; Südhof, T.C. Properties of native brain α-synuclein. Nature 2013, 498, E4. [Google Scholar] [CrossRef]
- Burré, J. The Synaptic Function of α-Synuclein. J. Parkinsons Dis 2015, 5, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Auluck, P.K.; Chan, H.Y.E.; Trojanowski, J.Q.; Lee, V.M.Y.; Bonini, N.M. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science 2002, 295, 865–868. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.E.; Dodson, M.W.; Jiang, C.; Cao, J.H.; Huh, J.R.; Seol, J.H.; Yoo, S.J.; Hay, B.A.; Guo, M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature 2006, 441, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Cackovic, J.; Gutierrez-Luke, S.; Call, G.B.; Juba, A.; O’Brien, S.; Jun, C.H.; Buhlman, L.M. Vulnerable Parkin Loss-of-Function Drosophila Dopaminergic Neurons Have Advanced Mitochondrial Aging, Mitochondrial Network Loss and Transiently Reduced Autophagosome Recruitment. Front. Cell. Neurosci. 2018, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, T.; Vilain, S.; Vints, K.; Gounko, N.; Verstreken, P.; Vandenberghe, W. Deficiency of parkin and PINK1 impairs age-dependent mitophagy in Drosophila. Elife 2018, 7. [Google Scholar] [CrossRef]
- Pickrell, A.M.; Youle, R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Crocker, A.; Shahidullah, M.; Levitan, I.B.; Sehgal, A. Identification of a neural circuit that underlies the effects of octopamine on sleep:wake behavior. Neuron 2010, 65, 670–681. [Google Scholar] [CrossRef]
- Canet-Avilés, R.M.; Wilson, M.A.; Miller, D.W.; Ahmad, R.; McLendon, C.; Bandyopadhyay, S.; Baptista, M.J.; Ringe, D.; Petsko, G.A.; Cookson, M.R. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. USA 2004, 101, 9103–9108. [Google Scholar] [CrossRef]
- Junn, E.; Jang, W.H.; Zhao, X.; Jeong, B.S.; Mouradian, M.M. Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J. Neurosci. Res. 2009, 87, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-J.; Park, Y.-J.; Hwang, I.-Y.; Youdim, M.B.H.; Park, K.-S.; Oh, Y.J. Nuclear translocation of DJ-1 during oxidative stress-induced neuronal cell death. Free Radic. Biol. Med. 2012, 53, 936–950. [Google Scholar] [CrossRef]
- Biosa, A.; Sandrelli, F.; Beltramini, M.; Greggio, E.; Bubacco, L.; Bisaglia, M. Recent findings on the physiological function of DJ-1: Beyond Parkinson’s disease. Neurobiol. Dis. 2017, 108, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Aradska, J.; Bulat, T.; Sialana, F.J.; Birner-Gruenberger, R.; Erich, B.; Lubec, G. Gel-free mass spectrometry analysis of Drosophila melanogaster heads. Proteomics 2015, 15, 3356–3360. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yang, Z.; Yue, Z.; Alvarez, J.D.; Sehgal, A. FOXO and insulin signaling regulate sensitivity of the circadian clock to oxidative stress. Proc. Natl. Acad. Sci. USA 2007, 104, 15899–15904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zordan, M.A.; Benna, C.; Mazzotta, G. Monitoring and analyzing Drosophila circadian locomotor activity. Methods Mol. Biol. 2007, 362, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Holtzman, D.M. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 2016, 354, 1004–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondratov, R.V.; Vykhovanets, O.; Kondratova, A.A.; Antoch, M.P. Antioxidant N-acetyl-L-cysteine ameliorates symptoms of premature aging associated with the deficiency of the circadian protein BMAL1. Aging 2009, 1, 979–987. [Google Scholar] [CrossRef] [Green Version]
- Musiek, E.S.; Lim, M.M.; Yang, G.; Bauer, A.Q.; Qi, L.; Lee, Y.; Roh, J.H.; Ortiz-Gonzalez, X.; Dearborn, J.T.; Culver, J.P.; et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J. Clin. Investig. 2013, 123, 5389–5400. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Moulik, M.; Fang, Z.; Saha, P.; Zou, F.; Xu, Y.; Nelson, D.L.; Ma, K.; Moore, D.D.; Yechoor, V.K. Bmal1 and β-cell clock are required for adaptation to circadian disruption, and their loss of function leads to oxidative stress-induced β-cell failure in mice. Mol. Cell. Biol. 2013, 33, 2327–2338. [Google Scholar] [CrossRef]
- Kim, J.; Jang, S.; Choi, M.; Chung, S.; Choe, Y.; Choe, H.K.; Son, G.H.; Rhee, K.; Kim, K. Abrogation of the Circadian Nuclear Receptor REV-ERBα Exacerbates 6-Hydroxydopamine-Induced Dopaminergic Neurodegeneration. Mol. Cells 2018, 41, 742–752. [Google Scholar] [CrossRef]
- Krishnan, N.; Davis, A.J.; Giebultowicz, J.M. Circadian regulation of response to oxidative stress in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2008, 374, 299–303. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, N.; Kretzschmar, D.; Rakshit, K.; Chow, E.; Giebultowicz, J.M. The circadian clock gene period extends healthspan in aging Drosophila melanogaster. Aging 2009, 1, 937–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, N.; Rakshit, K.; Chow, E.S.; Wentzell, J.S.; Kretzschmar, D.; Giebultowicz, J.M. Loss of circadian clock accelerates aging in neurodegeneration-prone mutants. Neurobiol. Dis. 2012, 45, 1129–1135. [Google Scholar] [CrossRef] [Green Version]
- Vaccaro, A.; Issa, A.-R.; Seugnet, L.; Birman, S.; Klarsfeld, A. Drosophila Clock Is Required in Brain Pacemaker Neurons to Prevent Premature Locomotor Aging Independently of Its Circadian Function. PLoS Genet. 2017, 13, e1006507. [Google Scholar] [CrossRef]
- Means, J.C.; Venkatesan, A.; Gerdes, B.; Fan, J.-Y.; Bjes, E.S.; Price, J.L. Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy. PLOS Genet. 2015, 11, e1005171. [Google Scholar] [CrossRef] [PubMed]
- Chong, F.P.; Ng, K.Y.; Koh, R.Y.; Chye, S.M. Tau Proteins and Tauopathies in Alzheimer’s Disease. Cell. Mol. Neurobiol. 2018, 38, 965–980. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Ayton, S.; Finkelstein, D.I.; Adlard, P.A.; Masters, C.L.; Bush, A.I. Tau protein: Relevance to Parkinson’s disease. Int. J. Biochem. Cell Biol. 2010, 42, 1775–1778. [Google Scholar] [CrossRef] [PubMed]
- Muskus, M.J.; Preuss, F.; Fan, J.-Y.; Bjes, E.S.; Price, J.L. Drosophila DBT Lacking Protein Kinase Activity Produces Long-Period and Arrhythmic Circadian Behavioral and Molecular Rhythms. Mol. Cell. Biol. 2007, 27, 8049–8064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barth, M.; Schultze, M.; Schuster, C.M.; Strauss, R. Circadian Plasticity in Photoreceptor Cells Controls Visual Coding Efficiency in Drosophila melanogaster. PLoS ONE 2010, 5, e9217. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, G.; Rossi, A.; Leonardi, E.; Mason, M.; Bertolucci, C.; Caccin, L.; Spolaore, B.; Martin, A.J.M.; Schlichting, M.; Grebler, R.; et al. Fly cryptochrome and the visual system. Proc. Natl. Acad. Sci. USA 2013, 110, 6163–6168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, R.J.H.; Furmston, R.; Williams, C.A.C.; Elliott, C.J.H. Neurophysiology of Drosophila Models of Parkinson’s Disease. Parkinson’s Dis. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Afsari, F.; Christensen, K.V.; Smith, G.P.; Hentzer, M.; Nippe, O.M.; Elliott, C.J.H.; Wade, A.R. Abnormal visual gain control in a Parkinson’s disease model. Hum. Mol. Genet. 2014, 23, 4465–4478. [Google Scholar] [CrossRef] [PubMed]
- Reichmann, H.; Brandt, M.D.; Klingelhoefer, L. The nonmotor features of Parkinson’s disease: Pathophysiology and management advances. Curr. Opin. Neurol. 2016, 29, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Obeso, J.A.; Halliday, G.M. Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 101–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seugnet, L.; Suzuki, Y.; Vine, L.; Gottschalk, L.; Shaw, P.J. D1 Receptor Activation in the Mushroom Bodies Rescues Sleep-Loss-Induced Learning Impairments in Drosophila. Curr. Biol. 2008, 18, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Faville, R.; Kottler, B.; Goodhill, G.J.; Shaw, P.J.; van Swinderen, B. How deeply does your mutant sleep? Probing arousal to better understand sleep defects in Drosophila. Sci. Rep. 2015, 5, 8454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilestro, G.F. Video tracking and analysis of sleep in Drosophila melanogaster. Nat. Protoc. 2012, 7, 995–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Mammalian Models | Intervention | Animal Model | Circadian Phenotype |
|---|---|---|---|
| ASO | Overexpression of wt α-syn in all brain regions | Mouse | Fragmented circadian rhythms and a reduced firing rate of SCN neurons during the day [31] |
| MitoPark | Deletion for mitochondrial transcription factor A in dopaminergic neurons | Mouse | Age-dependent rhythm decline and disturbed circadian activity rhythms under constant high light conditions [32] |
| MPTP-injection | Intraperitoneally and subcutaneously [33] Intraperitoneally [34] | Mouse | No significant changes in circadian parameters [33,34] |
| Not specified [35] | Mouse | Reduced amplitude in locomotor rhythm; altered clock gene expression in the SCN [35] | |
| Intravenously [36] | Dog | Circadian urine volume and vasopressin release alteration [36] | |
| Intramuscular [37] | Non-human Primates | Loss of circadian locomotor activity in the absence of light/dark cues [37] | |
| 6-OHDA-injection | Bilateral in the striatum [38] | Rat | Altered clock gene expression in the striatum [38] |
| Intracerebroventricular and unilateral infusion in medial forebrain bundle [39] | Rat | Disorganized wheel-running pattern in constant darkness and blunted PER2 expression rise in the dorsal striatum [39] | |
| Bilateral in the ventral tegmental area [40] | Rat | Reduced locomotor activity period in LD and longer activity rhythm periodicity under constant dim light [40] | |
| Bilateral in the striatum [41] | Rat | Decreased amplitude of the heart rate rhythm [41] | |
| Bilateral in the striatum [42] | Rat | Altered clock gene expression profile in SNC and in the striatum [42] |
| Fly Genetic PD Model | |||||
|---|---|---|---|---|---|
| Gene | Genetic Manipulation | PD Phenotype | Circadian Phenotype | ||
| Dopaminergic Cells Death | Protein Inclusions | Locomotor Deficit | |||
| hSNCA | wt-αS overexpression | Yes [99] | No [99] | Yes [99] | Altered locomotor activity profiles [96] |
| A53T overexpression | Yes [99] | Yes [99] | Yes [99] | n.d | |
| TP-αS (A30P, A56P, A76P) overexpression | Yes [96] | Yes [100] | Yes [100] | Circadian locomotor periodicity shift with aging [96] | |
| dPink1 | Loss of function | Yes [101] | n.d. | Yes [101] | Arrhythmic, hyperexcitability of l-LNvs neurons, day/night difference in RMP less pronounced [97] |
| Absence of circadian locomotor anticipatory activity in the morning in LD [98] | |||||
| dpark | Loss of function | Yes [102] | n.d. | Yes [102] | Weakly rhythmic, No difference in day/night SFR ratio [97] |
| Absence of circadian locomotor anticipatory activity in the morning in LD [98] | |||||
| ddj-1α | Loss of function | Yes [103] | n.d. | n.d. | n.d. |
| ddj-1β | Loss of function | No [104] | No [105] | Yes [105,106] | n.d. |
| ddj-1α; ddj-1β | Loss of function | No [107] | n.d. | Yes [108] | No evident abnormalities observed (this work) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Lazzari, F.; Bisaglia, M.; Zordan, M.A.; Sandrelli, F. Circadian Rhythm Abnormalities in Parkinson’s Disease from Humans to Flies and Back. Int. J. Mol. Sci. 2018, 19, 3911. https://doi.org/10.3390/ijms19123911
De Lazzari F, Bisaglia M, Zordan MA, Sandrelli F. Circadian Rhythm Abnormalities in Parkinson’s Disease from Humans to Flies and Back. International Journal of Molecular Sciences. 2018; 19(12):3911. https://doi.org/10.3390/ijms19123911
Chicago/Turabian StyleDe Lazzari, Federica, Marco Bisaglia, Mauro Agostino Zordan, and Federica Sandrelli. 2018. "Circadian Rhythm Abnormalities in Parkinson’s Disease from Humans to Flies and Back" International Journal of Molecular Sciences 19, no. 12: 3911. https://doi.org/10.3390/ijms19123911
APA StyleDe Lazzari, F., Bisaglia, M., Zordan, M. A., & Sandrelli, F. (2018). Circadian Rhythm Abnormalities in Parkinson’s Disease from Humans to Flies and Back. International Journal of Molecular Sciences, 19(12), 3911. https://doi.org/10.3390/ijms19123911