Biosynthesis of Metal Nanoparticles via Microbial Enzymes: A Mechanistic Approach
Abstract
:1. Background and Role of Microbial Enzymes in Metal Nanoparticle (MtNP) Biosynthesis
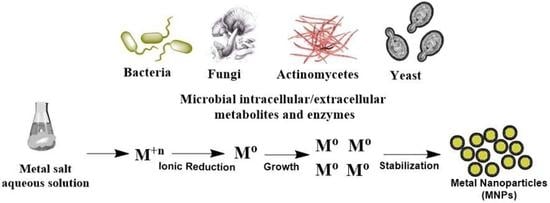
2. Biosynthesis of MtNPs by Microorganisms
3. Bacterial and Cyanobacterial Biosynthesis of MtNPs
4. Mycosynthesis of Nanoparticles
5. Algae as Biosynthesis Factories
6. Mechanisms of MtNP Synthesis by Microorganisms
7. Extracellular Enzymes
8. Intracellular Enzymes
9. Challenges and Limitation of MtNP Synthesis by Microorganisms: A Possible Solution
10. Conclusions and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- FutureMarketInsights Global Market for Metal & Metal Oxide Nanoparticles to Surge at More Than 10% CAGR. Available online: http://markets.businessinsider.com/news/stocks/Global-Market-forMetal-Metal-Oxide-Nanoparticles-to-Surge-at-More-Than-10CAGR-1001862836 (accessed on 21 November 2018).
- Mukherjee, S.; Vinothkumar, B.; Prashanthi, S.; Bangal, P.R.; Sreedhar, B.; Patra, C.R. Potential therapeutic and diagnostic applications of one-step in situ biosynthesized gold nanoconjugates (2-in-1 system) in cancer treatment. RSC Adv. 2013, 3, 2318–2329. [Google Scholar] [CrossRef]
- Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C 2015, 53, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Zia, N.; Ahmad, I.; Khalil, A.T.; Raza, A.; Ayaz, M.; Sadiq, A.; Ullah, F.; Shinwari, Z.K. Phyto-Therapeutic and Nanomedicinal Approaches to Cure Alzheimer’s Disease: Present Status and Future Opportunities. Front. Aging Neurosci. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Dasari, M.; Priyamvada, S.; Kotcherlakota, R.; Bollu, V.S.; Patra, C.R. A green chemistry approach for the synthesis of gold nanoconjugates that induce the inhibition of cancer cell proliferation through induction of oxidative stress and their in vivo toxicity study. J. Mater. Chem. B 2015, 3, 3820–3830. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Raza, A.; Khan, M.A.; Ahmad, I.; Islam, N.U.; Saravanan, M.; Ubaid, M.F.; Ali, M.; Shinwari, Z.K. Green synthesis of silver nanoparticles via plant extracts: beginning a new era in cancer theranostics. Nanomedicine 2016, 12, 3157–3177. [Google Scholar] [CrossRef] [PubMed]
- Salunke, G.R.; Ghosh, S.; Kumar, R.S.; Khade, S.; Vashisth, P.; Kale, T.; Chopade, S.; Pruthi, V.; Kundu, G.; Bellare, J.R. Rapid efficient synthesis and characterization of silver, gold, and bimetallic nanoparticles from the medicinal plant Plumbago zeylanica and their application in biofilm control. Int. J. Nanomed. 2014, 9, 2635. [Google Scholar]
- Barabadi, H.; Ovais, M.; Shinwari, Z.K.; Saravanan, M. Anti-cancer green bionanomaterials: present status and future prospects. Green Chem. Lett. Rev. 2017, 10, 285–314. [Google Scholar] [CrossRef]
- Emmanuel, R.; Saravanan, M.; Ovais, M.; Padmavathy, S.; Shinwari, Z.K.; Prakash, P. Antimicrobial efficacy of drug blended biosynthesized colloidal gold nanoparticles from Justicia glauca against oral pathogens: a nanoantibiotic approach. Microb. Pathog. 2017, 113, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Hulkoti, N.I.; Taranath, T. Biosynthesis of nanoparticles using microbes—A review. Colloids Surf. B 2014, 121, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Shi, J.; Wu, H.; Zhang, S.; Liu, H.; Zou, H.; Wu, Y.; Zhao, J.; Jiang, Z. In situ biosynthesis of ultrafine metal nanoparticles within a metal-organic framework for efficient heterogeneous catalysis. Nanotechnology 2017, 28, 365604. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Raza, A.; Naz, S.; Islam, N.U.; Khalil, A.T.; Ali, S.; Khan, M.A.; Shinwari, Z.K. Current state and prospects of the phytosynthesized colloidal gold nanoparticles and their applications in cancer theranostics. Appl. Microbiol. Biotechnol. 2017, 101, 3551–3565. [Google Scholar] [CrossRef] [PubMed]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of plant phytochemicals and microbial enzymes in biosynthesis of metallic nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.; Rakshith, D.; Kavitha, K.S.; Santosh, P.; Kavitha, H.U.; Rao, Y.; Satish, S. Plants: Emerging as nanofactories towards facile route in synthesis of nanoparticles. BioImpacts 2013, 3, 111. [Google Scholar] [PubMed]
- Kumari, R.; Barsainya, M.; Singh, D.P. Biogenic synthesis of silver nanoparticle by using secondary metabolites from Pseudomonas aeruginosa DM1 and its anti-algal effect on Chlorella vulgaris and Chlorella pyrenoidosa. Environ. Sci. Pollut. Res. 2017, 24, 4645–4654. [Google Scholar] [CrossRef] [PubMed]
- Patra, C.R.; Mukherjee, S.; Kotcherlakota, R. Biosynthesized silver nanoparticles: A step forward for cancer theranostics? Nanomedicine 2014, 9, 1445–1448. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Nethi, S.K.; Patra, C.R. Green synthesized gold nanoparticles for future biomedical applications. In Particulate Technology for Delivery of Therapeutics; Springer: Singapore, 2017; pp. 359–393. [Google Scholar]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Khamlich, S.; Maaza, M. Sageretia thea (Osbeck.) mediated synthesis of zinc oxide nanoparticles and its biological applications. Nanomedicine 2017, 12, 1767–1789. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Hassan, D.; Maaza, M. Sageretia thea (Osbeck.) modulated biosynthesis of NiO nanoparticles and their in vitro pharmacognostic, antioxidant and cytotoxic potential. Artif. Cells Nanomed. Biotechnol. 2018, 46, 838–852. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.; Love, A.; Sinitsyna, O.; Makarova, S.; Yaminsky, I.; Taliansky, M.; Kalinina, N. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar]
- Sintubin, L.; Verstraete, W.; Boon, N. Biologically produced nanosilver: Current state and future perspectives. Biotechnol. Bioeng. 2012, 109, 2422–2436. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Sushma, V.; Patra, S.; Barui, A.K.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Green chemistry approach for the synthesis and stabilization of biocompatible gold nanoparticles and their potential applications in cancer therapy. Nanotechnology 2012, 23, 455103. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Sau, S.; Madhuri, D.; Bollu, V.S.; Madhusudana, K.; Sreedhar, B.; Banerjee, R.; Patra, C.R. Green synthesis and characterization of monodispersed gold nanoparticles: Toxicity study, delivery of doxorubicin and its bio-distribution in mouse model. J. Biomed. Nanotechnol. 2016, 12, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 2014, 4, 316–335. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Wagh, P.; Wadhwani, S.; Gaidhani, S.; Kumbhar, A.; Bellare, J.; Chopade, B.A. Synthesis, optimization, and characterization of silver nanoparticles from Acinetobacter calcoaceticus and their enhanced antibacterial activity when combined with antibiotics. Int. J. Nanomed. 2013, 8, 4277. [Google Scholar] [Green Version]
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic silver nanoparticles: Synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Barik, S.K.; Mubarak Ali, D.; Prakash, P.; Pugazhendhi, A. Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb. Pathog. 2018, 116, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Klaus, T.; Joerger, R.; Olsson, E.; Granqvist, C.-G. Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. USA 1999, 96, 13611–13614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pugazhenthiran, N.; Anandan, S.; Kathiravan, G.; Prakash, N.K.U.; Crawford, S.; Ashokkumar, M. Microbial synthesis of silver nanoparticles by Bacillus sp. J. Nanopart. Res. 2009, 11, 1811. [Google Scholar] [CrossRef]
- Husseiny, M.; El-Aziz, M.A.; Badr, Y.; Mahmoud, M. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2007, 67, 1003–1006. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Guo, Z.; Zhang, Y.; Zhang, S.; Wang, J.; Gu, N. Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulata. Mater. Lett. 2007, 61, 3984–3987. [Google Scholar] [CrossRef]
- Dhandapani, P.; Siddarth, A.S.; Kamalasekaran, S.; Maruthamuthu, S.; Rajagopal, G. Bio-approach: Ureolytic bacteria mediated synthesis of ZnO nanocrystals on cotton fabric and evaluation of their antibacterial properties. Carbohyd. Polym. 2014, 103, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Selvarajan, E.; Mohanasrinivasan, V. Biosynthesis and characterization of ZnO nanoparticles using Lactobacillus plantarum VITES07. Mater. Lett. 2013, 112, 180–182. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Kirthi, A.V.; Marimuthu, S.; Santhoshkumar, T.; Bagavan, A.; Gaurav, K.; Karthik, L.; Rao, K.B. Novel microbial route to synthesize ZnO nanoparticles using Aeromonas hydrophila and their activity against pathogenic bacteria and fungi. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 90, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Rad, M.; Taran, M.; Alavi, M. Effect of Incubation Time, CuSO4 and Glucose Concentrations on Biosynthesis of Copper Oxide (CuO) Nanoparticles with Rectangular Shape and Antibacterial Activity: Taguchi Method Approach. Nano Biomed. Eng. 2018, 10, 25–33. [Google Scholar] [CrossRef]
- Fatemi, M.; Mollania, N.; Momeni-Moghaddam, M.; Sadeghifar, F. Extracellular biosynthesis of magnetic iron oxide nanoparticles by Bacillus cereus strain HMH1: Characterization and in vitro cytotoxicity analysis on MCF-7 and 3T3 cell lines. J. Biotechnol. 2018, 270, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nair, B.; Pradeep, T. Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst. Growth Des. 2002, 2, 293–298. [Google Scholar] [CrossRef]
- Lengke, M.F.; Fleet, M.E.; Southam, G. Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver (I) nitrate complex. Langmuir 2007, 23, 2694–2699. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Berthold, D.; Puranik, P.; Gantar, M. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol. Rep. 2015, 5, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Tomer, A.K.; Rahi, T.; Neelam, D.K.; Dadheech, P.K. Cyanobacterial extract-mediated synthesis of silver nanoparticles and their application in ammonia sensing. Int. Microbiol. 2018. [Google Scholar] [CrossRef]
- Satapathy, S.; Shukla, S.P. Application of a marine cyanobacterium Phormidium fragile for green synthesis of silver nanoparticles. Indian J. Biotechnol. 2017, 16, 110–113. [Google Scholar]
- Sonker, A.S.; Pathak, J.; Kannaujiya, V.; Sinha, R. Characterization and in vitro antitumor, antibacterial and antifungal activities of green synthesized silver nanoparticles using cell extract of Nostoc sp. strain HKAR-2. Can. J. Biotechnol. 2017, 1, 26–37. [Google Scholar] [CrossRef]
- Bakir, E.; Younis, N.; Mohamed, M.; El Semary, N. Cyanobacteria as Nanogold Factories: Chemical and Anti-Myocardial Infarction Properties of Gold Nanoparticles Synthesized by Lyngbya majuscula. Mar. Drugs 2018, 16, 217. [Google Scholar] [CrossRef] [PubMed]
- Molnár, Z.; Bódai, V.; Szakacs, G.; Erdélyi, B.; Fogarassy, Z.; Sáfrán, G.; Varga, T.; Kónya, Z.; Tóth-Szeles, E.; Szűcs, R. Green synthesis of gold nanoparticles by thermophilic filamentous fungi. Sci. Rep. 2018, 8, 3943. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhou, L.; Riaz Rajoka, M.S.; Yan, L.; Jiang, C.; Shao, D.; Zhu, J.; Shi, J.; Huang, Q.; Yang, H. Fungal silver nanoparticles: Synthesis, application and challenges. Crit. Rev. Biotechnol. 2018, 38, 817–835. [Google Scholar] [CrossRef] [PubMed]
- Alghuthaymi, M.A.; Almoammar, H.; Rai, M.; Said-Galiev, E.; Abd-Elsalam, K.A. Myconanoparticles: Synthesis and their role in phytopathogens management. Biotechnol. Biotechnol. Eq. 2015, 29, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Castro-Longoria, E.; Vilchis-Nestor, A.R.; Avalos-Borja, M. Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora crassa. Colloids Surf. B 2011, 83, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, X.; Wang, K.; Yang, X. Different active biomolecules involved in biosynthesis of gold nanoparticles by three fungus species. J. Biomed. Nanotechnol. 2011, 7, 245–254. [Google Scholar] [CrossRef] [PubMed]
- AbdelRahim, K.; Mahmoud, S.Y.; Ali, A.M.; Almaary, K.S.; Mustafa, A.E.-Z.M.; Husseiny, S.M. Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer. Saudi J. Biol. Sci. 2017, 24, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Jalal, M.; Ansari, M.; Alzohairy, M.; Ali, S.; Khan, H.; Almatroudi, A.; Raees, K. Biosynthesis of Silver Nanoparticles from Oropharyngeal Candida glabrata Isolates and Their Antimicrobial Activity against Clinical Strains of Bacteria and Fungi. Nanomaterials 2018, 8, 586. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, V.; Kataru, B.A.S.; Sravani, N.; Vigneshwari, T.; Panneerselvam, A.; Rajeswari, V.D. Biosynthesis of Zinc oxide nanoparticles using culture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies. OpenNano 2018. [Google Scholar] [CrossRef]
- Vijayanandan, A.S.; Balakrishnan, R.M. Biosynthesis of cobalt oxide nanoparticles using endophytic fungus Aspergillus nidulans. J. Environ. Manag. 2018, 218, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Sanaeimehr, Z.; Javadi, I.; Namvar, F. Antiangiogenic and antiapoptotic effects of green-synthesized zinc oxide nanoparticles using Sargassum muticum algae extraction. Cancer Nanotechnol. 2018, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Maceda, A.F.; Ouano, J.J.S.; Que, M.C.O.; Basilia, B.A.; Potestas, M.J.; Alguno, A.C. Controlling the Absorption of Gold Nanoparticles via Green Synthesis Using Sargassum crassifolium Extract. In Key Engineering Materials; Trans Tech Publ: Clausthal-Zellerfeld, Germany, 2018; pp. 44–48. [Google Scholar]
- Gu, H.; Chen, X.; Chen, F.; Zhou, X.; Parsaee, Z. Ultrasound-assisted biosynthesis of CuO-NPs using brown alga Cystoseira trinodis: Characterization, photocatalytic AOP, DPPH scavenging and antibacterial investigations. Ultrason. Sonochem. 2018, 41, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Koopi, H.; Buazar, F. A novel one-pot biosynthesis of pure alpha aluminum oxide nanoparticles using the macroalgae Sargassum ilicifolium: A green marine approach. Ceram. Int. 2018, 44, 8940–8945. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Mahadevan, A.; Sathishkumar, M.; Pavagadhi, S.; Balasubramanian, R. Biosynthesis of Au (0) from Au (III) via biosorption and bioreduction using brown marine alga Turbinaria conoides. Chem. Eng. J. 2011, 167, 223–227. [Google Scholar] [CrossRef]
- Ramakrishna, M.; Babu, D.R.; Gengan, R.M.; Chandra, S.; Rao, G.N. Green synthesis of gold nanoparticles using marine algae and evaluation of their catalytic activity. J. Nanostruct. Chem. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Swaminathan, S.; Murugesan, S.; Damodarkumar, S.; Dhamotharan, R.; Bhuvaneshwari, S. Synthesis and characterization of gold nanoparticles from alga Acanthophora spicifera (VAHL) Boergesen. Int. J. Nanosci. Nanotechnol. 2011, 2, 85–94. [Google Scholar]
- Ghodake, G.; Lee, D.S. Biological synthesis of gold nanoparticles using the aqueous extract of the brown algae Laminaria japonica. J. Nanoelectron. Optoelectron. 2011, 6, 268–271. [Google Scholar] [CrossRef]
- Govindaraju, K.; Basha, S.K.; Kumar, V.G.; Singaravelu, G. Silver, gold and bimetallic nanoparticles production using single-cell protein (Spirulina platensis) Geitler. J. Mater. Sci. 2008, 43, 5115–5122. [Google Scholar] [CrossRef]
- Wypij, M.; Golinska, P.; Dahm, H.; Rai, M. Actinobacterial-mediated synthesis of silver nanoparticles and their activity against pathogenic bacteria. IET Nanobiotechnol. 2016, 11, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Vashisth, P.; Pruthi, V.; Chopade, B.A. Kinetics of synthesis of gold nanoparticles by Acinetobacter sp. SW30 isolated from environment. Indian J. Microbiol. 2016, 56, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Prema, P.; Iniya, P.; Immanuel, G. Microbial mediated synthesis, characterization, antibacterial and synergistic effect of gold nanoparticles using Klebsiella pneumoniae (MTCC-4030). RSC Adv. 2016, 6, 4601–4607. [Google Scholar] [CrossRef]
- Manikprabhu, D.; Cheng, J.; Chen, W.; Sunkara, A.K.; Mane, S.B.; Kumar, R.; Hozzein, W.N.; Duan, Y.-Q.; Li, W.-J. Sunlight mediated synthesis of silver nanoparticles by a novel actinobacterium (Sinomonas mesophila MPKL 26) and its antimicrobial activity against multi drug resistant Staphylococcus aureus. J. Photochem. Photobiol. B 2016, 158, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Syed, B.; Prasad, N.M.; Satish, S. Endogenic mediated synthesis of gold nanoparticles bearing bactericidal activity. J. Microsc. Ultrastruct. 2016, 4, 162–166. [Google Scholar] [PubMed]
- Gan, L.; Zhang, S.; Zhang, Y.; He, S.; Tian, Y. Biosynthesis, characterization and antimicrobial activity of silver nanoparticles by a halotolerant Bacillus endophyticus SCU-L. Prep. Biochem. Biotechnol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Vijayabharathi, R.; Sathya, A.; Gopalakrishnan, S. Extracellular biosynthesis of silver nanoparticles using Streptomyces griseoplanus SAI-25 and its antifungal activity against Macrophomina phaseolina, the charcoal rot pathogen of sorghum. Biocatal. Agric. Biotechnol. 2018, 14, 166–171. [Google Scholar] [CrossRef]
- Ranjani, A.; Gopinath, P.M.; Ananth, S.; Narchonai, G.; Santhanam, P.; Thajuddin, N.; Dhanasekaran, D. Multidimensional dose–response toxicity exploration of silver nanoparticles from Nocardiopsis flavascens RD30. Appl. Nanosci. 2018, 8, 699–713. [Google Scholar] [CrossRef]
- Bing, W.; Sun, H.; Wang, F.; Song, Y.; Ren, J. Hydrogen-producing Hyperthermophilic Bacteria Synthesized Size-controllable Fine Gold Nanoparticles with Excellence for Eradicating Biofilm and Antibacterial Applications. J. Mater. Chem. B 2018. [Google Scholar] [CrossRef]
- Lv, Q.; Zhang, B.; Xing, X.; Zhao, Y.; Cai, R.; Wang, W.; Gu, Q. Biosynthesis of copper nanoparticles using Shewanella loihica PV-4 with antibacterial activity: Novel approach and mechanisms investigation. J. Hazard. Mater. 2018, 347, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Kalathil, S.; Shi, L.; Alharbi, O.; Wang, P. Synthesis of ultra-small platinum, palladium and gold nanoparticles by Shewanella loihica PV-4 electrochemically active biofilms and their enhanced catalytic activities. J. Saudi Chem. Soc. 2018. [Google Scholar] [CrossRef]
- Jafari, M.; Rokhbakhsh-Zamin, F.; Shakibaie, M.; Moshafi, M.H.; Ameri, A.; Rahimi, H.R.; Forootanfar, H. Cytotoxic and antibacterial activities of biologically synthesized gold nanoparticles assisted by Micrococcus yunnanensis strain J2. Biocatal. Agric. Biotechnol. 2018. [Google Scholar] [CrossRef]
- Camas, M.; Camas, A.S.; Kyeremeh, K. Extracellular Synthesis and Characterization of Gold Nanoparticles Using Mycobacterium sp. BRS2A-AR2 Isolated from the Aerial Roots of the Ghanaian Mangrove Plant, Rhizophora racemosa. Indian J. Microbiol. 2018, 58, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Oza, G.; Pandey, S.; Sharon, M. Biogenic fabrication of gold nanoparticles using Halomonas salina. J. Microbiol. Biotechnol. 2017, 2, 485–492. [Google Scholar]
- Kobashigawa, J.M.; Robles, C.A.; Ricci, M.L.M.; Carmarán, C.C. Influence of strong bases on the synthesis of silver nanoparticles (AgNPs) using the ligninolytic fungi Trametes trogii. Saudi J.Biol. Sci. 2018. [Google Scholar] [CrossRef]
- Elamawi, R.M.; Al-Harbi, R.E.; Hendi, A.A. Biosynthesis and characterization of silver nanoparticles using Trichoderma longibrachiatum and their effect on phytopathogenic fungi. Egypt. J. Biol. Pest Control 2018, 28, 28. [Google Scholar] [CrossRef]
- Tripathi, R.M.; Shrivastav, B.R.; Shrivastav, A. Antibacterial and catalytic activity of biogenic gold nanoparticles synthesised byTrichoderma harzianum. IET Nanobiotechnol. 2018, 12, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.-A.; Hamzah, H.; Maaroof, M. Analyzing formation of silver nanoparticles from the filamentous fungus Fusarium oxysporum and their antimicrobial activity. Turk. J. Biol. 2018, 42, 54–62. [Google Scholar] [CrossRef]
- El Domany, E.B.; Essam, T.M.; Ahmed, A.E.; Farghali, A.A. Biosynthesis Physico-Chemical Optimization of Gold Nanoparticles as Anti-Cancer and Synergetic Antimicrobial Activity Using Pleurotus ostreatus Fungus. J. Appl. Pharm. Sci. Vol. 2018, 8, 119–128. [Google Scholar]
- Singh, P.S.; Vidyasagar, G. Biosynthesis of antibacterial silver nano-particles from Aspergillus terreus. World News Nat. Sci. 2018, 16, 117–124. [Google Scholar]
- Mohanta, Y.K.; Nayak, D.; Biswas, K.; Singdevsachan, S.K.; Abd_Allah, E.F.; Hashem, A.; Alqarawi, A.A.; Yadav, D.; Mohanta, T.K. Silver Nanoparticles Synthesized Using Wild Mushroom Show Potential Antimicrobial Activities against Food Borne Pathogens. Molecules 2018, 23, 655. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Arokiyaraj, S.; Lakshmi, T.; Pugazhendhi, A. Synthesis of silver nanoparticles from Phenerochaete chrysosporium (MTCC-787) and their antibacterial activity against human pathogenic bacteria. Microb. Pathog. 2018, 117, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Neethu, S.; Midhun, S.J.; Sunil, M.; Soumya, S.; Radhakrishnan, E.; Jyothis, M. Efficient visible light induced synthesis of silver nanoparticles by Penicillium polonicum ARA 10 isolated from Chetomorpha antennina and its antibacterial efficacy against Salmonella enterica serovar Typhimurium. J. Photochem. Photobiol. B 2018, 180, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Spagnoletti, F.N.; Spedalieri, C.; Kronberg, F.; Giacometti, R. Extracellular biosynthesis of bactericidal Ag/AgCl nanoparticles for crop protection using the fungus Macrophomina phaseolina. J. Environ. Manag. 2019, 231, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.A.; da CSO Cunha, M.; da Frota, S.M.; Mallmann, E.J.; Freire, T.M.; Costa, L.S.; Paula, A.J.; Menezes, E.A.; Fechine, P.B. Biogenic synthesis of multifunctional silver nanoparticles from Rhodotorula glutinis and Rhodotorula mucilaginosa: Antifungal, catalytic and cytotoxicity activities. World J. Microbiol. Biotechnol. 2018, 34, 127. [Google Scholar] [CrossRef] [PubMed]
- Popli, D.; Anil, V.; Subramanyam, A.B.; Rao, S.N.; Rai, R.V.; Govindappa, M. Endophyte fungi, Cladosporium species-mediated synthesis of silver nanoparticles possessing in vitro antioxidant, anti-diabetic and anti-Alzheimer activity. Artif. Cells Nanomed. 2018. [Google Scholar] [CrossRef] [PubMed]
- Joshi, C.G.; Danagoudar, A.; Poyya, J.; Kudva, A.K.; Dhananjaya, B. Biogenic synthesis of gold nanoparticles by marine endophytic fungus-Cladosporium cladosporioides isolated from seaweed and evaluation of their antioxidant and antimicrobial properties. Process Biochem. 2017, 63, 137–144. [Google Scholar]
- Farsi, M.; Farokhi, S. Biosynthesis of Antibacterial Silver Nanoparticles by Endophytic Fungus Nemania sp. Isolated From Taxus baccata L.(Iranian Yew). Zahedan J. Res. Med. Sci. 2018, 20. [Google Scholar] [CrossRef]
- Subramaniyan, S.A.; Sheet, S.; Vinothkannan, M.; Yoo, D.J.; Lee, Y.S.; Belal, S.A.; Shim, K.S. One-Pot Facile Synthesis of Pt Nanoparticles Using Cultural Filtrate of Microgravity Simulated Grown P. chrysogenum and Their Activity on Bacteria and Cancer Cells. J. Nanosci. Nanotechnol. 2018, 18, 3110–3125. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Qu, Y.; Pei, X.; Li, S.; You, S.; Wang, J.; Zhang, Z.; Zhou, J. Catalytic reduction of 4-nitrophenol using gold nanoparticles biosynthesized by cell-free extracts of Aspergillus sp. WL-Au. J. Hazard. Mater. 2017, 321, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, M.; Anand, K.V.; Govindaraju, K.; Tamilselvan, S.; Kumar, V.G. Seaweed Sargassum wightii mediated preparation of zirconia (ZrO2) nanoparticles and their antibacterial activity against gram positive and gram negative bacteria. Microb. Pathog. 2018, 124, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Lan, C.Q. Mechanism of light-dependent biosynthesis of silver nanoparticles mediated by cell extract of Neochloris oleoabundans. Colloids Surf. B 2018. [Google Scholar] [CrossRef] [PubMed]
- González-Ballesteros, N.; Prado-López, S.; Rodriguez-Gonzalez, J.; Lastra, M.; Rodríguez-Argüelles, M. Green synthesis of gold nanoparticles using brown algae cystoseira baccata: Its activity in colon cancer cells. Colloids Surf. B 2017, 153, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Pytlik, N.; Kaden, J.; Finger, M.; Naumann, J.; Wanke, S.; Machill, S.; Brunner, E. Biological synthesis of gold nanoparticles by the diatom Stephanopyxis turris and in vivo SERS analyses. Algal Res. 2017, 28, 9–15. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef]
- Arsiya, F.; Sayadi, M.H.; Sobhani, S. Green synthesis of palladium nanoparticles using Chlorella vulgaris. Mater. Lett. 2017, 186, 113–115. [Google Scholar] [CrossRef]
- Ramkumar, V.S.; Pugazhendhi, A.; Gopalakrishnan, K.; Sivagurunathan, P.; Saratale, G.D.; Dung, T.N.B.; Kannapiran, E. Biofabrication and characterization of silver nanoparticles using aqueous extract of seaweed Enteromorpha compressa and its biomedical properties. Biotechnol. Rep. 2017, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vanlalveni, C.; Rajkumari, K.; Biswas, A.; Adhikari, P.P.; Lalfakzuala, R.; Rokhum, L. Green Synthesis of Silver Nanoparticles Using Nostoc linckia and its Antimicrobial Activity: A Novel Biological Approach. Bionanoscience 2018, 8, 624–631. [Google Scholar] [CrossRef]
- Zada, S.; Ahmad, A.; Khan, S.; Yu, X.; Chang, K.; Iqbal, A.; Ahmad, A.; Ullah, S.; Raza, M.; Khan, A. Biogenic synthesis of silver nanoparticles using extracts of Leptolyngbya JSC-1 that induce apoptosis in HeLa cell line and exterminate pathogenic bacteria. Artif. Cells Nanomed. Biotechnol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.; Bhuvaneswari, S.; Sivamurugan, V. Green synthesis, characterization of silver nanoparticles of a marine red alga Spyridia fusiformis and their antibacterial activity. Int. J. Pharm. Pharm. Sci. 2017, 9, 192–197. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Yang, Q.; Lan, K.; Yan, Z.; Chen, J. Eco-friendly intracellular microalgae synthesis of fluorescent CdSe QDs as a sensitive nanoprobe for determination of imatinib. Sens. Actuators B Chem. 2018, 263, 625–633. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M.; Alharbi, R.M.; Alkhulaifi, M.M. Biosynthesis of silver nanoparticles by using of the marine brown alga Padina pavonia and their characterization. Saudi J. Biol. Sci. 2018. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Salmani, N.; Heidari, A.; Rezaei, M.R. Bio-synthesis of palladium nanoparticle using Spirulina platensis alga extract and its application as adsorbent. Surf. Interfaces 2018, 10, 136–143. [Google Scholar] [CrossRef]
- Sharma, M.; Behl, K.; Nigam, S.; Joshi, M. TiO2-GO nanocomposite for photocatalysis and environmental applications: A green synthesis approach. Vacuum 2018, 156, 434–439. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloids Interface Sci. 2010, 156. [Google Scholar] [CrossRef] [PubMed]
- Subbaiya, R.; Saravanan, M.; Priya, A.R.; Shankar, K.R.; Selvam, M.; Ovais, M.; Balajee, R.; Barabadi, H. Biomimetic synthesis of silver nanoparticles from Streptomyces atrovirens and their potential anticancer activity against human breast cancer cells. IET Nanobiotechnol. 2017, 11, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Bose, D.; Chatterjee, S. Biogenic synthesis of silver nanoparticles using guava (Psidium guajava) leaf extract and its antibacterial activity against Pseudomonas aeruginosa. Appl. Nanosci. 2016, 6, 895–901. [Google Scholar] [CrossRef]
- Mukherjee, S.; Patra, C.R. Biologically synthesized metal nanoparticles: Recent advancement and future perspectives in cancer theranostics. Future Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Abyaneh, M.K.; Gosavi, S.; Kulkarni, S.K.; Pasricha, R.; Ahmad, A.; Khan, M. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol. Lett. 2007, 29, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Ansary, A.A.; Ahmad, A.; Khan, M. Extracellular biosynthesis of CdSe quantum dots by the fungus, Fusarium oxysporum. J. Biomed. Nanotechnol. 2007, 3, 190–194. [Google Scholar] [CrossRef]
- Ingle, A.; Gade, A.; Pierrat, S.; Sonnichsen, C.; Rai, M. Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Curr. Nanosci. 2008, 4, 141–144. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Alves, O.L.; De Souza, G.I.; Esposito, E. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J. Nanobiotechnol. 2005, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Ahmad, A.; Khan, M.I.; Sastry, M.; Kumar, R. Extracellular biosynthesis of bimetallic Au–Ag alloy nanoparticles. Small 2005, 1, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Karbasian, M.; Atyabi, S.; Siadat, S.; Momen, S.; Norouzian, D. Optimizing nano-silver formation by Fusarium oxysporum PTCC 5115 employing response surface methodology. Am. J. Agric. Biol. Sci. 2008, 3, 433–437. [Google Scholar]
- Ahmad, A.; Mukherjee, P.; Mandal, D.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Enzyme mediated extracellular synthesis of CdS nanoparticles by the fungus, Fusarium oxysporum. J. Am. Chem. Soc. 2002, 124, 12108–12109. [Google Scholar] [CrossRef] [PubMed]
- Basavaraja, S.; Balaji, S.; Lagashetty, A.; Rajasab, A.; Venkataraman, A. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater. Res. Bull. 2008, 43, 1164–1170. [Google Scholar] [CrossRef]
- Ingle, A.; Rai, M.; Gade, A.; Bawaskar, M. Fusarium solani: A novel biological agent for the extracellular synthesis of silver nanoparticles. J. Nanopart. Res. 2009, 11, 2079. [Google Scholar] [CrossRef]
- Balaji, D.; Basavaraja, S.; Deshpande, R.; Mahesh, D.B.; Prabhakar, B.; Venkataraman, A. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf. B 2009, 68, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Gade, A.; Bonde, P.; Ingle, A.; Marcato, P.; Duran, N.; Rai, M. Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J. Biobased Mater. Biol. 2008, 2, 243–247. [Google Scholar] [CrossRef]
- Bhainsa, K.C.; D’souza, S. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf. B 2006, 47, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Kathiresan, K.; Manivannan, S.; Nabeel, M.; Dhivya, B. Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids Surf. B 2009, 71, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Shaligram, N.S.; Bule, M.; Bhambure, R.; Singhal, R.S.; Singh, S.K.; Szakacs, G.; Pandey, A. Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process. Biochem. 2009, 44, 939–943. [Google Scholar] [CrossRef]
- Singaravelu, G.; Arockiamary, J.; Kumar, V.G.; Govindaraju, K. A novel extracellular synthesis of monodisperse gold nanoparticles using marine alga, Sargassum wightii Greville. Colloids Surf. B 2007, 57, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Lengke, M.F.; Fleet, M.E.; Southam, G. Morphology of gold nanoparticles synthesized by filamentous cyanobacteria from gold (I)− thiosulfate and gold (III)− chloride complexes. Langmuir 2006, 22, 2780–2787. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.R.; Yong, P.; Macaskie, L.E. Enzymatic recovery of elemental palladium by using sulfate-reducing bacteria. Appl. Environ. Microbiol. 1998, 64, 4607–4609. [Google Scholar] [PubMed]
- Kashefi, K.; Lovley, D.R. Reduction of Fe (III), Mn (IV), and toxic metals at 100 C by Pyrobaculum islandicum. Appl. Environ. Microbiol. 2000, 66, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Lu, Z.; Cui, X.; Qiao, Y.; Guo, J.; Anderson, J.M.; Li, C.M. Extracellular microbial synthesis of biocompatible CdTe quantum dots. Acta Biomater. 2010, 6, 3534–3541. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Jiang, H.; Liu, X.; Wang, E. Biosynthesis of gold nanoparticles assisted by Escherichia coli DH5α and its application on direct electrochemistry of hemoglobin. Electrochem. Commun. 2007, 9, 1165–1170. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Babu, R.S.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B 2008, 65, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Han, J.; Choi, H.; Hur, H.-G. Effects of temperature and dissolved oxygen on Se (IV) removal and Se (0) precipitation by Shewanella sp. HN-41. Chemosphere 2007, 68, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- Juibari, M.M.; Abbasalizadeh, S.; Jouzani, G.S.; Noruzi, M. Intensified biosynthesis of silver nanoparticles using a native extremophilic Ureibacillus thermosphaericus strain. Mater. Lett. 2011, 65, 1014–1017. [Google Scholar] [CrossRef]
- Sneha, K.; Sathishkumar, M.; Mao, J.; Kwak, I.; Yun, Y.-S. Corynebacterium glutamicum-mediated crystallization of silver ions through sorption and reduction processes. Chem. Eng. J. 2010, 162, 989–996. [Google Scholar] [CrossRef]
- Suresh, A.K.; Pelletier, D.A.; Wang, W.; Broich, M.L.; Moon, J.-W.; Gu, B.; Allison, D.P.; Joy, D.C.; Phelps, T.J.; Doktycz, M.J. Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium Shewanella oneidensis. Acta Biomater. 2011, 7, 2148–2152. [Google Scholar] [CrossRef] [PubMed]
- Lengke, M.F.; Ravel, B.; Fleet, M.E.; Wanger, G.; Gordon, R.A.; Southam, G. Mechanisms of gold bioaccumulation by filamentous cyanobacteria from gold (III)− chloride complex. Environ. Sci. Technol. 2006, 40, 6304–6309. [Google Scholar] [CrossRef] [PubMed]
- Vigneshwaran, N.; Kathe, A.A.; Varadarajan, P.; Nachane, R.P.; Balasubramanya, R. Biomimetics of silver nanoparticles by white rot fungus, Phaenerochaete chrysosporium. Colloids Surf. B 2006, 53, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Vigneshwaran, N.; Ashtaputre, N.; Varadarajan, P.; Nachane, R.; Paralikar, K.; Balasubramanya, R. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater. Lett. 2007, 61, 1413–1418. [Google Scholar] [CrossRef]
- Senapati, S.; Mandal, D.; Ahmad, A. Fungus mediated synthesis of silver nanoparticles: A novel biological approach. Indian J. Phys. A 2004, 78, 101. [Google Scholar]
- Fayaz, A.M.; Balaji, K.; Kalaichelvan, P.; Venkatesan, R. Fungal based synthesis of silver nanoparticles—An effect of temperature on the size of particles. Colloids Surf. B 2009, 74, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, M.; Joshi, S.; Kumar, A.R.; Zinjarde, S.; Kulkarni, S. Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Mater. Lett. 2009, 63, 1231–1234. [Google Scholar] [CrossRef]
- Konishi, Y.; Ohno, K.; Saitoh, N.; Nomura, T.; Nagamine, S.; Hishida, H.; Takahashi, Y.; Uruga, T. Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae. J. Biotechnol. 2007, 128, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Sinha, A.; Khare, S.K. Mercury bioaccumulation and simultaneous nanoparticle synthesis by Enterobacter sp. cells. Bioresour. Technol. 2011, 102, 4281–4284. [Google Scholar] [CrossRef] [PubMed]
- Babu, M.G.; Gunasekaran, P. Production and structural characterization of crystalline silver nanoparticles from Bacillus cereus isolate. Colloids Surf. B 2009, 74, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; Deepak, V.; Pandian, S.R.K.; Kottaisamy, M.; BarathManiKanth, S.; Kartikeyan, B.; Gurunathan, S. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf. B 2010, 77, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Ramani, R.; Srinivas, V.; Sastry, M. Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology 2003, 14, 824. [Google Scholar] [CrossRef]
- Gericke, M.; Pinches, A. Biological synthesis of metal nanoparticles. Hydrometallurgy 2006, 83, 132–140. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomedicine 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Dauthal, P.; Mukhopadhyay, M. Noble metal nanoparticles: Plant-mediated synthesis, mechanistic aspects of synthesis, and applications. Ind. Eng. Chem. Res. 2016, 55, 9557–9577. [Google Scholar] [CrossRef]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular biosynthesis of monodisperse gold nanoparticles by a novel extremophilic actinomycete, Thermomonospora sp. Langmuir 2003, 19, 3550–3553. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.; Alam, M.; Kumar, R. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: A novel biological approach to nanoparticle synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Ramani, R.; Parischa, R.; Ajayakumar, P.; Alam, M. Bioreduction of AuCl4−ions by the fungus, Verticillium sp. and surface trapping of the gold nanoparticles formed. Angew. Chem. Int. Ed. 2001, 40, 3585–3588. [Google Scholar] [CrossRef]
- Southam, G.; Beveridge, T.J. The occurrence of sulfur and phosphorus within bacterially derived crystalline and pseudocrystalline octahedral gold formed in vitro. Geochim. Cosmochim. Acta 1996, 60, 4369–4376. [Google Scholar] [CrossRef]
- Sanghi, R.; Verma, P.; Puri, S. Enzymatic formation of gold nanoparticles using Phanerochaete chrysosporium. Adv. Chem. Eng. Sci. 2011, 1, 154. [Google Scholar] [CrossRef]
- Konishi, Y.; Tsukiyama, T.; Tachimi, T.; Saitoh, N.; Nomura, T.; Nagamine, S. Microbial deposition of gold nanoparticles by the metal-reducing bacterium Shewanella algae. Electrochim. Acta 2007, 53, 186–192. [Google Scholar] [CrossRef]
- Mody, V.V.; Siwale, R.; Singh, A.; Mody, H.R. Introduction to metallic nanoparticles. J. Pharm. Bioallied Sci. 2010, 2, 282. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Nethi, S.K.; Mukherjee, S.; Veeriah, V.; Barui, A.K.; Chatterjee, S.; Patra, C.R. Bioconjugated gold nanoparticles accelerate the growth of new blood vessels through redox signaling. Chem. Commun. 2014, 50, 14367–14370. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.-H.; Shanmugam, V.; Yeh, C.-S. Nanoparticle biosynthesis using unicellular and subcellular supports. NPG Asia Mater. 2015, 7, e209. [Google Scholar] [CrossRef]
- Korbekandi, H.; Iravani, S.; Abbasi, S. Production of nanoparticles using organisms. Crit. Rev. Biotechnol. 2009, 29, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Patra, C.R. Therapeutic application of anti-angiogenic nanomaterials in cancers. Nanoscale 2016, 8, 12444–12470. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Lin, Z.; Gu, P.; Zhou, J.; Yao, B.; Chen, G.; Fu, J. Extracellular biosynthesis of monodispersed gold nanoparticles by a SAM capping route. J. Nanopart. Res. 2009, 11, 279–288. [Google Scholar] [CrossRef]
- Balakrishnan, S.; Mukherjee, S.; Das, S.; Bhat, F.A.; Raja, S.P.; Patra, C.R.; Arunakaran, J. Gold nanoparticles-conjugated quercetin induces apoptosis via inhibition of EGFR/PI3K/Akt-mediated pathway in breast cancer cell lines (MCF-7 and MDA-MB-231). Cell Biochem. Funct. 2017, 35, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-W.; Rawn, C.J.; Rondinone, A.J.; Love, L.J.; Roh, Y.; Everett, S.M.; Lauf, R.J.; Phelps, T.J. Large-scale production of magnetic nanoparticles using bacterial fermentation. J. Ind. Microbiol. Biotechnol. 2010, 37, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Korbekandi, H.; Iravani, S.; Abbasi, S. Optimization of biological synthesis of silver nanoparticles using Lactobacillus casei subsp. casei. J. Chem. Technol. Biotechnol. 2012, 87, 932–937. [Google Scholar] [CrossRef]

| Serial Number | Microorganisms | Nanoparticle | Size/Shape | Application | Reference |
|---|---|---|---|---|---|
| Bacteria | |||||
| 1 | Actinobacter | Ag | 13.2 nm/Spherical | Antibacterial | [65] |
| 2 | Acinetobacter | Au | 19 nm/Spherical-triangular-polyhedral | - | [66] |
| 3 | Klebsiella pneumonia | Au | 10–15 nm/Spherical | Antibacterial | [67] |
| 4 | Sinomonas mesophila | Ag | 4–50 nm/Spherical | Antibacterial | [68] |
| 5 | Pseudomonas fluorescens | Au | 5–50 nm/Spherical | Antibacterial | [69] |
| 6 | Bacillus endophyticus | Ag | 5.1 nm/Spherical | Antimicrobial | [70] |
| 7 | Bacillus brevis | Ag | 41–68 nm/Spherical | Antibacterial | [30] |
| 8 | Streptomycesgriseoplanus | Ag | 19.5–20.9 nm/Spherical | Antifungal | [71] |
| 9 | Nocardiopsis flavascens | Ag | 5 and 50/Spherical | Cytotoxicity | [72] |
| 10 | Caldicellulosiruptor changbaiensis | Au | <20 nm/Spherical | Antibacterial, Antibiofilm | [73] |
| 11 | Shewanella loihica | Cu | 10–16 nm/Spherical | Antibacterial | [74] |
| 12 | Shewanella loihica | Pt | 1–10 nm/Spherical | Dye degradation | [75] |
| 13 | Shewanella loihica | Pd | 1–12 nm/Spherical | Dye degradation | [75] |
| 14 | Shewanella loihica | Au | 2–15 nm/Spherical | Dye degradation | [75] |
| 15 | Micrococcus yunnanensis | Au | 53.8 nm/Spherical | Antibacterial, Anticancer | [76] |
| 16 | Mycobacterium sp. | Au | 5–55 nm/Spherical | Anticancer | [77] |
| 17 | Halomonas salina | Au | 30–100 nm/Spherical | - | [78] |
| Fungi | |||||
| 18 | Aspergillus niger | ZnO | 53–69 nm/Spherical | Antibacterial Dye degradation | [54] |
| 19 | Trametes trogii | Ag | 5–65 nm/Spherical- Ellipsoidal | - | [79] |
| 20 | Trichoderma longibrachiatum | Ag | 10 nm/Spherical | Antifungal against phyto-pathogenic fungi | [80] |
| 21 | Trichoderma harzianum | Au | 32–44 nm/Spherical | Antibacterial, Dye degradation | [81] |
| 22 | Fusarium oxysporum | Ag | 21.3–37 nm/Spherical | Antimicrobial | [82] |
| 23 | Pleurotus ostreatus | Au | 10–30 nm/Spherical | Antimicrobial, Anticancer | [83] |
| 24 | Aspergillus terreus | Ag | 16–57 nm/Spherical | Antibacterial | [84] |
| 25 | Ganoderma sessiliforme | Ag | ~45 nm/Spherical | Antibacterial, Antioxidant, Anticancer | [85] |
| 26 | Phenerochaete chrysosporium | Ag | 34–90 nm/Spherical-Oval | Antibacterial | [86] |
| 27 | Penicillium polonicum | Ag | 10–15 nm/Spherical | Antibacterial | [87] |
| 28 | Candida glabrata | Ag | 2–15 nm/Spherical | Antibacterial | [53] |
| 29 | Macrophomina phaseolina | Ag/AgCl | 5–30 nm/Spherical | Antibacterial | [88] |
| 30 | Aspergillus nidulans | CoO | 20.29 nm/Spinel | - | [55] |
| 31 | Rhodotorula glutinis | Ag | 15.45 nm/Spherical | Antifungal, Dye degradation, Cytotoxicity | [89] |
| 32 | Rhodotorulamucilaginosa | Ag | 13.70 nm/Spherical | Antifungal, Dye degradation, Cytotoxicity | [89] |
| 33 | Cladosporium sp. | Ag | 24 nm/Spherical | Antioxidant, Antidiabetic, Anti-Alzheimer | [90] |
| 34 | Cladosporium cladosporioides | Au | 60 nm/Round | Antioxidant, Antibacterial | [91] |
| 35 | Nemania sp. | Ag | 33.52 nm/Spherical | Antibacterial | [92] |
| 36 | Penicillium chrysogenum | Pt | 5–40 nm/Spherical | Cytotoxicity | [93] |
| 37 | Aspergillus sp. | Au | 2.5–6.7 nm/Spherical | Nitrophenol reduction | [94] |
| 38 | Rhizopus stolonifer | Ag | 2.86 nm/Spherical | - | [52] |
| Algae/Cyanobacteria | |||||
| 39 | Sargassum wightii | ZrO2 | 18 nm/Spherical | Antibacterial | [95] |
| 40 | Neochloris oleoabundans | Ag | 40 nm/Spherical | Antibacterial | [96] |
| 41 | Cystoseira baccata | Au | 8.4 nm/Spherical | Anticancer | [97] |
| 42 | Stephanopyxis turris | Au | 10–30 nm/Spherical | - | [98] |
| 43 | Galaxaura elongate | Au | 3.85–77 nm/Spherical-rods-triangular | Antibacterial | [99] |
| 44 | Chlorella vulgaris | Pd | 5–20 nm nm/Spherical | - | [100] |
| 45 | Enteromorpha compressa | Ag | 4–24 nm/Spherical | Antimicrobial, Anticancer | [101] |
| 46 | Nostoc linckia | Ag | 5–60 nm/Spherical | Antibacterial | [102] |
| 47 | Nostoc sp | Ag | 51–100 nm/Spherical | Spherical | [45] |
| 48 | Leptolyngbya | Ag | 5–50 nm/Spherical | Antibacterial, Anticancer | [103] |
| 49 | Spyridia fusiformis | Ag | 5–50 nm/Spherical | Antibacterial | [104] |
| 50 | Chlorella pyrenoidosa | CdSe QD | 4–5 nm | Imatinib sensing | [105] |
| 52 | Sargassum ilicifolium | Al2O3 | 20 nm/Spherical | - | [59] |
| 53 | Padina pavonia | Ag | 49.58–86.37 nm/spherical-triangular-rectangle-polyhedral-hexagonal | - | [106] |
| 53 | Spirulina platensis | Pd | 10–20 nm/Spherical | Adsorbent | [107] |
| 54 | Chlorella pyrenoidosa | TiO2 | 50 nm/Spherical | Dye degradation | [108] |
| Source of Extracellular Enzymes (Bacterial Species) | Nature of Organism | Metals Used | Shape | Size (nm) | Temperature (°C) | Reference |
| Desulfovibrio desulfuricans | G−ive Bacteria | Pd | Round | 50 | 25 | [129] |
| Pyrobaculum islandicum | G−ive Rods | U, Tc, Cr, Co, Mn | Round | NA | 100 | [130] |
| Escherichia coli | G−ive Bacteria | CdTe | Round | 2–3.2 | 37 | [131] |
| Escherichia coli | G−ive Bacteria | Au | Hexagonal, Triangle | 20–30 | 37 | [132] |
| Bacillus licheniformis | G+ive mesophilic bacteria | Ag | NA | 50 | 37 | [133] |
| Shewanella species | Marine Bacteria | Se | Round | 181 | 30 | [134] |
| Ureibacillus thermosphaericus | G+ive Bacteria | Au | NA | 50–70 | 60–80 | [135] |
| Corynebacterium glutamicum | G+ive Bacteria | Ag | Irregular | 5–50 | 30 | [136] |
| Rhodopseudomonas capsulate | Phototrophic Bacteria | Au | Round | 10–20 | 30 | [34] |
| Pseudomonas aeruginosa | G−ive Bacteria | Au | NA | 15–30 | 37 | [33] |
| Shewanella Oneidensis | Facultative Bacteria | Au | Round | 12 | 30 | [137] |
| Fungi and Algae Species | ||||||
| Plectonema boryanum UTEX 485 | Filamentous Fungi | Au | Octahedral | 10 nm–6 µm | 25 | [138] |
| Phaenerochaete chrysosporium | Fungi | Ag | Pyramidal | 50–200 | 37 | [139] |
| Aspergillus flavus | Fungi | Ag | Round | 8.92 | 25 | [140] |
| Yeast | Fungi | Au, Ag | Polygonal | 9–25 | 30 | [131] |
| Fusarium oxysporum | Ascomycete fungus | Alloy of Au–Ag | Round | 8–14 | 25 | [117] |
| Sargassum wightii | Macro-algae | Au | Planar | 8–12 | NA | [127] |
| Neurospora crassa | Bread mold | Au–Ag, Au | Round | 20–50 | 28 | [50] |
| Verticillium sp. | Fungi | Ag | Round | 25–32 | 25 | [141] |
| Aspergillus fumigatus | Fungi | Ag | Round | 5–25 | 25 | [124] |
| Trichoderma viride | Fungi | Ag | NA | 2–4 | 10–40 | [142] |
| Yarrowia lipolytica | Fungi | Au | Triangles | 15 | 30 | [143] |
| Source of Intracellular Enzyme (Bacterial Species) | Nature of Microb. | Metals used | Shape | Size(nm) | Temperature (°C) | Reference |
| Shewanella algae | G−ive marine bacteria | Pt | NA | 5 | 25 | [144] |
| Enterobacter species | Anaerobic G−ive Bacilli | Hg | Round | 2–5 | 30 | [145] |
| Bacillus cereus | G+ive Bacteria | Ag | Round | 4–5 | 37 | [146] |
| Brevibacterium casei | Actinomycetales Bacteria | Ag, Au | Roud | 10–50 | 37 | [147] |
| Rhodococcus sp. | Actinobacteria | Au | Round | 8-12 | NA | [148] |
| Fungi and Algae Species | ||||||
| Plectonema boryanum | Algae | Au | Cubic | <10–25 | 25–100 | [128] |
| Neurospora crassa | Bread mold | Au–Ag, Au | Round | 32 | 28 | [50] |
| Verticillum luteoalbum | Ascomycota Fungi | Au | NA | NA | 37 | [149] |
| Candida utilis | Fungus | Au | NA | NA | 25 | [149] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovais, M.; Khalil, A.T.; Ayaz, M.; Ahmad, I.; Nethi, S.K.; Mukherjee, S. Biosynthesis of Metal Nanoparticles via Microbial Enzymes: A Mechanistic Approach. Int. J. Mol. Sci. 2018, 19, 4100. https://doi.org/10.3390/ijms19124100
Ovais M, Khalil AT, Ayaz M, Ahmad I, Nethi SK, Mukherjee S. Biosynthesis of Metal Nanoparticles via Microbial Enzymes: A Mechanistic Approach. International Journal of Molecular Sciences. 2018; 19(12):4100. https://doi.org/10.3390/ijms19124100
Chicago/Turabian StyleOvais, Muhammad, Ali Talha Khalil, Muhammad Ayaz, Irshad Ahmad, Susheel Kumar Nethi, and Sudip Mukherjee. 2018. "Biosynthesis of Metal Nanoparticles via Microbial Enzymes: A Mechanistic Approach" International Journal of Molecular Sciences 19, no. 12: 4100. https://doi.org/10.3390/ijms19124100
APA StyleOvais, M., Khalil, A. T., Ayaz, M., Ahmad, I., Nethi, S. K., & Mukherjee, S. (2018). Biosynthesis of Metal Nanoparticles via Microbial Enzymes: A Mechanistic Approach. International Journal of Molecular Sciences, 19(12), 4100. https://doi.org/10.3390/ijms19124100