Macrophage Migration Inhibitory Factor (MIF) Inhibition in a Murine Model of Bleomycin-Induced Pulmonary Fibrosis
Abstract
:1. Introduction
2. Results
2.1. MIF and Its Two Main Receptors, CD74 and CXCR4, Are Upregulated in Lungs from Patients with IPF Associated with PH (IPF-PH)
2.2. MIF, CD74, and CXCR4 Protein Levels Are Increased in Lungs from Bleomycin-Injected Mice
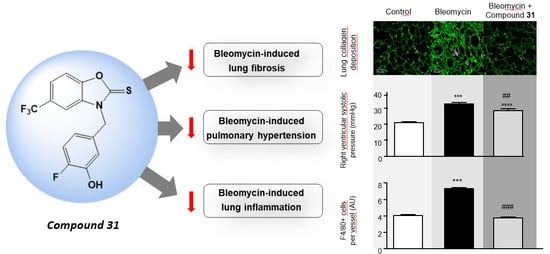
2.3. Chronic Treatment with ISO-1 and Compound 31 Attenuates Extracellular Matrix Deposition in Lungs of Bleomycin-Injected Mice
2.4. Daily Treatment with ISO-1 and Compound 31 Prevents the Development of Pulmonary Hypertension (PH) in Lungs of Bleomycin-Injected Mice
2.5. Chronic Treatment with ISO-1 and Compound 31 Decreases Perivascular Macrophage Accumulation in Lungs of Bleomycin-Injected Mice
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Study Protocol
4.3. Animals and Hemodynamic Measurements
4.4. Immunostaining, Immunofluorescence, and Confocal Analyses
4.5. Second Harmonic Generation (SHG) Microscopy
4.6. Western Blot Analysis and ELISA
4.7. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AU | arbitrary unit |
| BLM | bleomycin |
| BAL | bronchoalveolar lavage |
| DAPI | 4′,6-diamidino-2-phenylindole |
| H&E | hematoxylin and eosin |
| IPF | idiopathic pulmonary fibrosis |
| IL | interleukin |
| Ki | inhibition constant |
| PBMC | peripheral blood mononuclear cell |
| LV | left ventricle |
| α-SMA | α-smooth muscle actin |
| MIF | macrophage migration inhibitory factor |
| MCT | monocrotaline |
| PBS | phosphate-buffered saline |
| PH | pulmonary hypertension |
| PAH | pulmonary arterial hypertension |
| RV | right ventricle |
| RVH | right ventricular hypertrophy |
| S | septum |
| ISO-1 | (S,R)-3-(4-hydroxyphenyl)-4,5-dihydro-5-isoxazole acetic acid methyl ester |
| 4-HPP | 4-hydroxyphenylpyruvate |
| 31 | N-(3-hydroxy-4-fluorobenzyl)-5-trifluoromethylbenzoxazol-2-thione 31 |
References
- Guenther, A.; Krauss, E.; Tello, S.; Wagner, J.; Paul, B.; Kuhn, S.; Maurer, O.; Heinemann, S.; Costabel, U.; Barbero, M.A.N.; et al. The European IPF registry (eurIPFreg): Baseline characteristics and survival of patients with idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [PubMed]
- Kropski, J.A.; Lawson, W.E.; Young, L.R.; Blackwell, T.S. Genetic studies provide clues on the pathogenesis of idiopathic pulmonary fibrosis. Dis. Model. Mech. 2013, 6, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Bargagli, E.; Olivieri, C.; Nikiforakis, N.; Cintorino, M.; Magi, B.; Perari, M.G.; Vagaggini, C.; Spina, D.; Prasse, A.; Rottoli, P. Analysis of macrophage migration inhibitory factor (MIF) in patients with idiopathic pulmonary fibrosis. Respir. Physiol. Neurobiol. 2009, 167, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, C.; Bargagli, E.; Inghilleri, S.; Campo, I.; Cintorino, M.; Rottoli, P. Macrophage migration inhibitory factor in lung tissue of idiopathic pulmonary fibrosis patients. Exp. Lung Res. 2016, 42, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Tanino, Y.; Makita, H.; Miyamoto, K.; Betsuyaku, T.; Ohtsuka, Y.; Nishihira, J.; Nishimura, M. Role of macrophage migration inhibitory factor in bleomycin-induced lung injury and fibrosis in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L156–L162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corallo, C.; Paulesu, L.; Cutolo, M.; Ietta, F.; Carotenuto, C.; Mannelli, C.; Romagnoli, R.; Nuti, R.; Giordano, N. Serum levels, tissue expression and cellular secretion of macrophage migration inhibitory factor in limited and diffuse systemic sclerosis. Clin. Exp. Rheumatol. 2015, 33, 98S–S105. [Google Scholar] [CrossRef]
- Le Hiress, M.; Akagah, B.; Bernadat, G.; Tu, L.; Thuillet, R.; Huertas, A.; Phan, C.; Fadel, E.; Simonneau, G.; Humbert, M.; et al. Design, Synthesis, and Biological Activity of New N-(Phenylmethyl)-benzoxazol-2-thiones as Macrophage Migration Inhibitory Factor (MIF) Antagonists: Efficacies in Experimental Pulmonary Hypertension. J. Med. Chem. 2018, 61, 2725–2736. [Google Scholar] [CrossRef] [PubMed]
- Le Hiress, M.; Tu, L.; Ricard, N.; Phan, C.; Thuillet, R.; Fadel, E.; Dorfmuller, P.; Montani, D.; de Man, F.; Humbert, M.; et al. Proinflammatory Signature of the Dysfunctional Endothelium in Pulmonary Hypertension. Role of the Macrophage Migration Inhibitory Factor/CD74 Complex. Am. J. Respir. Crit. Care Med. 2015, 192, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Bonniaud, P.; Fabre, A.; Frossard, N.; Guignabert, C.; Inman, M.; Kuebler, W.M.; Maes, T.; Shi, W.; Stampfli, M.; Uhlig, S.; et al. Optimising experimental research in respiratory diseases: An ERS statement. Eur. Respir. J. 2018, 51, 1702133. [Google Scholar] [CrossRef] [PubMed]
- Moeller, A.; Ask, K.; Warburton, D.; Gauldie, J.; Kolb, M. The bleomycin animal model: A useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int. J. Biochem. Cell. Biol. 2008, 40, 362–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borzone, G.; Moreno, R.; Urrea, R.; Meneses, M.; Oyarzun, M.; Lisboa, C. Bleomycin-induced chronic lung damage does not resemble human idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2001, 163, 1648–1653. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, C.J.; Ruhrmund, D.W.; Pan, L.; Seiwert, S.D.; Kossen, K. Antifibrotic activities of pirfenidone in animal models. Eur. Respir. Rev. 2011, 20, 85–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wollin, L.; Maillet, I.; Quesniaux, V.; Holweg, A.; Ryffel, B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J. Pharmacol. Exp. Ther. 2014, 349, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Farkas, L.; Farkas, D.; Ask, K.; Moller, A.; Gauldie, J.; Margetts, P.; Inman, M.; Kolb, M. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J. Clin. Investig. 2009, 119, 1298–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Shen, M.; Xu, M.; Liu, L.L.; Luo, Y.; Xu, D.Q.; Wang, Y.X.; Liu, M.L.; Liu, Y.; Dong, H.Y.; et al. Role of macrophage migration inhibitory factor in the proliferation of smooth muscle cell in pulmonary hypertension. Mediators Inflamm. 2012, 2012, 840737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Talwar, A.; Tsang, D.; Bruchfeld, A.; Sadoughi, A.; Hu, M.; Omonuwa, K.; Cheng, K.F.; Al-Abed, Y.; Miller, E.J. Macrophage migration inhibitory factor mediates hypoxia-induced pulmonary hypertension. Mol. Med. 2012, 18, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Izbicki, G.; Segel, M.J.; Christensen, T.G.; Conner, M.W.; Breuer, R. Time course of bleomycin-induced lung fibrosis. Int. J. Exp. Pathol. 2002, 83, 111–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef] [PubMed]
- Colombat, M.; Mal, H.; Groussard, O.; Capron, F.; Thabut, G.; Jebrak, G.; Brugiere, O.; Dauriat, G.; Castier, Y.; Leseche, G.; et al. Pulmonary vascular lesions in end-stage idiopathic pulmonary fibrosis: Histopathologic study on lung explant specimens and correlations with pulmonary hemodynamics. Hum. Pathol. 2007, 38, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Gunther, S.; Fagone, P.; Jalce, G.; Atanasov, A.; Guignabert, C.; Nicoletti, F. Role of MIF and D-DT in immune-inflammatory, autoimmune, and chronic respiratory diseases: From pathogenic factors to therapeutic targets. Drug Discov. Today 2018. [Google Scholar] [CrossRef] [PubMed]
- Bruchfeld, A.; Carrero, J.J.; Qureshi, A.R.; Lindholm, B.; Barany, P.; Heimburger, O.; Hu, M.; Lin, X.; Stenvinkel, P.; Miller, E.J. Elevated serum macrophage migration inhibitory factor (MIF) concentrations in chronic kidney disease (CKD) are associated with markers of oxidative stress and endothelial activation. Mol. Med. 2009, 15, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Bai, Y.; Wu, L.; Hong, W.; Liang, Y.; Chen, B.; Bai, Y. Inhibition of Macrophage Migration Inhibitory Factor Protects against Inflammation and Matrix Deposition in Kidney Tissues after Injury. Mediators Inflamm. 2016, 2016, 2174682. [Google Scholar] [CrossRef] [PubMed]
- Ningyan, G.; Xu, Y.; Hongfei, S.; Jingjing, C.; Min, C. The role of macrophage migration inhibitory factor in mast cell-stimulated fibroblast proliferation and collagen production. PLoS ONE 2015, 10, e0122482. [Google Scholar] [CrossRef] [PubMed]
- Bossini-Castillo, L.; Campillo-Davo, D.; Lopez-Isac, E.; Carmona, F.D.; Simeon, C.P.; Carreira, P.; Callejas-Rubio, J.L.; Castellvi, I.; Fernandez-Nebro, A.; Rodriguez-Rodriguez, L.; et al. An MIF Promoter Polymorphism Is Associated with Susceptibility to Pulmonary Arterial Hypertension in Diffuse Cutaneous Systemic Sclerosis. J. Rheumatol. 2017, 44, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.; Sun, S.; Al-Abed, Y. MIF, a controversial cytokine: A review of structural features, challenges, and opportunities for drug development. Expert Opin. Ther. Targets 2016, 20, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Pantouris, G.; Syed, M.A.; Fan, C.; Rajasekaran, D.; Cho, T.Y.; Rosenberg, E.M., Jr.; Bucala, R.; Bhandari, V.; Lolis, E.J. An Analysis of MIF Structural Features that Control Functional Activation of CD74. Chem. Biol. 2015, 22, 1197–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajasekaran, D.; Groning, S.; Schmitz, C.; Zierow, S.; Drucker, N.; Bakou, M.; Kohl, K.; Mertens, A.; Lue, H.; Weber, C.; et al. Macrophage Migration Inhibitory Factor-CXCR4 Receptor Interactions: Evidence for Partial Allosteric Agonism in Comparison with CXCL12 Chemokine. J. Biol. Chem. 2016, 291, 15881–15895. [Google Scholar] [CrossRef] [PubMed]
- Al-Abed, Y.; VanPatten, S. MIF as a disease target: ISO-1 as a proof-of-concept therapeutic. Future Med. Chem. 2011, 3, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Cisneros, J.A.; Robertson, M.J.; Valhondo, M.; Jorgensen, W.L. Irregularities in enzyme assays: The case of macrophage migration inhibitory factor. Bioorg. Med. Chem. Lett. 2016, 26, 2764–2767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi-Parmar, V.; Jorgensen, W.L. Advances and Insights for Small Molecule Inhibition of Macrophage Migration Inhibitory Factor. J. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Kleemann, R.; Hausser, A.; Geiger, G.; Mischke, R.; Burger-Kentischer, A.; Flieger, O.; Johannes, F.J.; Roger, T.; Calandra, T.; Kapurniotu, A.; et al. Intracellular action of the cytokine MIF to modulate AP-1 activity and the cell cycle through Jab1. Nature 2000, 408, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Guignabert, C.; Phan, C.; Seferian, A.; Huertas, A.; Tu, L.; Thuillet, R.; Sattler, C.; Le Hiress, M.; Tamura, Y.; Jutant, E.M.; et al. Dasatinib induces lung vascular toxicity and predisposes to pulmonary hypertension. J. Clin. Investig. 2016, 126, 3207–3218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huertas, A.; Tu, L.; Thuillet, R.; Le Hiress, M.; Phan, C.; Ricard, N.; Nadaud, S.; Fadel, E.; Humbert, M.; Guignabert, C. Leptin signalling system as a target for pulmonary arterial hypertension therapy. Eur. Respir. J. 2015, 45, 1066–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, C.; Jutant, E.M.; Tu, L.; Thuillet, R.; Seferian, A.; Montani, D.; Huertas, A.; Bezu, J.V.; Breijer, F.; Vonk Noordegraaf, A.; et al. Dasatinib increases endothelial permeability leading to pleural effusion. Eur. Respir. J. 2018, 51, 1701096. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Phan, C.; Tu, L.; Le Hiress, M.; Thuillet, R.; Jutant, E.M.; Fadel, E.; Savale, L.; Huertas, A.; Humbert, M.; et al. Ectopic upregulation of membrane-bound IL6R drives vascular remodeling in pulmonary arterial hypertension. J. Clin. Investig. 2018, 128, 1956–1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, L.; De Man, F.S.; Girerd, B.; Huertas, A.; Chaumais, M.C.; Lecerf, F.; Francois, C.; Perros, F.; Dorfmuller, P.; Fadel, E.; et al. A critical role for p130Cas in the progression of pulmonary hypertension in humans and rodents. Am. J. Respir. Crit. Care Med. 2012, 186, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, T.; Odin, C.; Le Grand, Y.; Gailhouste, L.; Turlin, B.; Ezan, F.; Desille, Y.; Baffet, G.; Guyader, D. A robust collagen scoring method for human liver fibrosis by second harmonic microscopy. Opt. Express 2010, 18, 25794–25807. [Google Scholar] [CrossRef] [PubMed]
- De Man, F.S.; Tu, L.; Handoko, M.L.; Rain, S.; Ruiter, G.; Francois, C.; Schalij, I.; Dorfmuller, P.; Simonneau, G.; Fadel, E.; et al. Dysregulated renin-angiotensin-aldosterone system contributes to pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2012, 186, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Dewachter, L.; Gore, B.; Fadel, E.; Dartevelle, P.; Simonneau, G.; Humbert, M.; Eddahibi, S.; Guignabert, C. Autocrine fibroblast growth factor-2 signaling contributes to altered endothelial phenotype in pulmonary hypertension. Am. J. Respir. Cell. Mol. Biol. 2011, 45, 311–322. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Günther, S.; Bordenave, J.; Hua-Huy, T.; Nicco, C.; Cumont, A.; Thuillet, R.; Tu, L.; Quatremarre, T.; Guilbert, T.; Jalce, G.; et al. Macrophage Migration Inhibitory Factor (MIF) Inhibition in a Murine Model of Bleomycin-Induced Pulmonary Fibrosis. Int. J. Mol. Sci. 2018, 19, 4105. https://doi.org/10.3390/ijms19124105
Günther S, Bordenave J, Hua-Huy T, Nicco C, Cumont A, Thuillet R, Tu L, Quatremarre T, Guilbert T, Jalce G, et al. Macrophage Migration Inhibitory Factor (MIF) Inhibition in a Murine Model of Bleomycin-Induced Pulmonary Fibrosis. International Journal of Molecular Sciences. 2018; 19(12):4105. https://doi.org/10.3390/ijms19124105
Chicago/Turabian StyleGünther, Sven, Jennifer Bordenave, Thông Hua-Huy, Carole Nicco, Amélie Cumont, Raphaël Thuillet, Ly Tu, Timothée Quatremarre, Thomas Guilbert, Gaël Jalce, and et al. 2018. "Macrophage Migration Inhibitory Factor (MIF) Inhibition in a Murine Model of Bleomycin-Induced Pulmonary Fibrosis" International Journal of Molecular Sciences 19, no. 12: 4105. https://doi.org/10.3390/ijms19124105
APA StyleGünther, S., Bordenave, J., Hua-Huy, T., Nicco, C., Cumont, A., Thuillet, R., Tu, L., Quatremarre, T., Guilbert, T., Jalce, G., Batteux, F., Humbert, M., Savale, L., Guignabert, C., & Dinh-Xuan, A. -T. (2018). Macrophage Migration Inhibitory Factor (MIF) Inhibition in a Murine Model of Bleomycin-Induced Pulmonary Fibrosis. International Journal of Molecular Sciences, 19(12), 4105. https://doi.org/10.3390/ijms19124105