Nanofiltration and Tight Ultrafiltration Membranes for the Recovery of Polyphenols from Agro-Food By-Products
Abstract
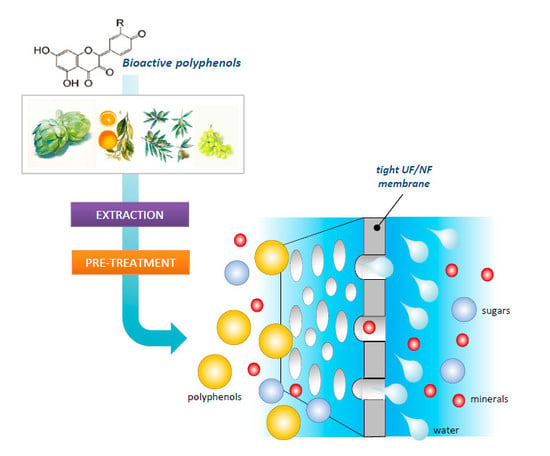
:1. Introduction
2. Extraction Methodologies of Polyphenols (PPs)
3. Separation and Purification Methodologies of PPs
4. General Aspects of Microfiltration (MF), Ultrafiltration (UF) and Nanofiltration (NF) Processes
5. Recovery of PPs by Tight UF and NF Membranes
5.1. Effect of Pre-Treatment on the Membrane Performance
5.1.1. Olive Mill Wastewaters (OMWs)
5.1.2. Artichoke Wastewaters
5.1.3. Citrus by-Products
5.2. Effect of Operating Conditions on the Membrane Performance
5.3. Effect of Molecular Weight Cut-off (MWCO) on the Membrane Performance
5.4. Effect of Membrane Material on the Membrane Performance
6. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| ASE | Accelerated solvent extraction |
| COD | Chemical oxygen demand |
| HVED | High voltage electrical discharges |
| MAE | Microwave assisted extraction |
| MF | Microfiltration |
| MWCO | Molecular weight cut-off |
| NF | Nanofiltration |
| OMWs | Olive mill wastewaters |
| PA | Polyamide |
| PEF | Pulsed electric fields |
| PES | Polyethersulfone |
| POH | pulsed ohmic heating |
| PPs | Polyphenols |
| PS | Polysulfone |
| PVDF | Polyvinylidene fluoride |
| RO | Reverse osmosis |
| SbFE | Subcritical fluid extraction |
| SCFE | Supercritical fluid extraction |
| TAA | Total antioxidant activity |
| TMP | Transmembrane pressure |
| TOC | Total organic carbon |
| TSS | Total suspended solids |
| UAE | Ultrasound-assisted extraction |
| UF | Ultrafiltration |
References
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Tapiero, H.; Tew, K.D.; Nguyen Ba, G.; Mathé, G. Polyphenols: Do they play a role in the preventions of human pathologies? Biomed. Pharmacother. 2002, 56, 200–2007. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [PubMed]
- Petti, S.; Scully, C. Polyphenols, oral health and disease: A review. J. Dent. 2009, 37, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Tripoli, E.; Gianmmanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef] [PubMed]
- El Gharras, H. Polyphenols: Food sources, properties and applications—A review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 1, 2073S–2085S. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Castro-Muñoz, R.; Cassano, A.; Conidi, C. Recovery of high-added-value compounds from food waste by membrane technology. In Membrane Technologies for Biorefining; Figoli, A., Cassano, A., Basile, A., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 189–215. ISBN 978-0-08-100451-7. [Google Scholar]
- Castro-Muñoz, R.; Barragán-Huerta, B.E.; Fíla, V.; Denis, P.C.; Ruby-Figueroa, R. Current role of membrane technology: From the treatment of agro-industrial by-products up to valorization of valuable compounds. Waste Biomass Valoriz. 2017, 1–17. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Yáñez-Fernández, J.; Fíla, V. Phenolic compounds recovered from agro-food by-products using membrane technologies: An overview. Food Chem. 2016, 213, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Morais, S.; Delerue-Matos, C. Environment-friendly techniques for extraction of bioactive compounds from fruits. In Soft Chemistry and Food Fermentation—Handbook of Food Bioengineering; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: London, UK, 2017; Volume 3, pp. 21–48. ISBN 978-0-12-811412-4. [Google Scholar]
- Shilpi, A.; Shivhare, U.S.; Basu, S. Supercritical CO2 extraction of compounds with antioxidant activity from fruits and vegetables waste—A review. Focus Mod. Food Ind. 2013, 2, 43–62. [Google Scholar]
- Brianceau, S.; Turk, M.; Vitrac, X.; Vorobiev, E. Combined densification and pulsed electric field treatment for selective polyphenols recovery from fermented grape pomace. Innov. Food Sci. Emerg. Technol. 2015, 29, 2–8. [Google Scholar] [CrossRef]
- Barba, F.J.; Brianceau, S.; Turk, M.; Boussetta, N.; Vorobiev, E. Effect of alternative physical treatments (Ultrasounds, pulsed electric fields, and high-voltage electrical discharges) on selective recovery of bio-compounds from fermented grape pomace. Food Bioprocess Technol. 2015, 8, 1139–1148. [Google Scholar] [CrossRef]
- El Darra, N.; Grimi, N.; Vorobiev, E.; Louka, N.; Maroun, R. Extraction of polyphenols from red grape pomace assisted by pulsed ohmic heating. Food Bioprocess Technol. 2013, 6, 1281–1289. [Google Scholar] [CrossRef]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Bleve, M.; Ciurlia, L.; Erroi, E.; Lionetto, G.; Longo, L.; Rescio, L.; Schettino, T.; Vasapollo, G. An innovative method for the purification of anthocyanins from grape skin extracts by using liquid and sub-critical carbon dioxide. Sep. Purif. Technol. 2008, 64, 192–197. [Google Scholar] [CrossRef]
- Pascual-Martí, M. Supercritical fluid extraction of resveratrol from grape skin of Vitis vinifera and determination by HPLC. Talanta 2001, 54, 735–740. [Google Scholar] [CrossRef]
- Corrales, M.; García, A.F.; Butz, P.; Tauscher, B. Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. J. Food Eng. 2009, 90, 415–421. [Google Scholar] [CrossRef]
- Rajha, H.N.; Ziegler, W.; Louka, N.; Hobaika, Z.; Vorobiev, E.; Boechzelt, H.G.; Maroun, R.G. Effect of the drying process on the intensification of phenolic compounds recovery from grape pomace using accelerated solvent extraction. Int. J. Mol. Sci. 2014, 15, 18640–18658. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.P.; Chang, Y.; Tan, Z.Y.; Li, F.F. A novel combined process for extracting, separating and recovering flavonoids from flos sophorae immaturus. Sep. Purif. Technol. 2017, 172, 422–432. [Google Scholar] [CrossRef]
- Wong Paz, J.E.; Muñiz Márquez, D.B.; Martínez Ávila, G.C.G.; Belmares Cerda, R.E.; Aguilar, C.N. Ultrasound-assisted extraction of polyphenols from native plants in the Mexican desert. Ultrason. Sonochem. 2014, 22, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Escobar, C.A.; Luque de Castro, M.D. Towards a comprehensive exploitation of citrus. Trends Food Sci. Technol. 2014, 39, 63–75. [Google Scholar] [CrossRef]
- Desai, M.; Parikh, J.; Parikh, P.A. Extraction of natural products using microwaves as a heat source. Sep. Purif. Rev. 2010, 39, 1–32. [Google Scholar] [CrossRef]
- Mendes, M.; Carvalho, A.P.; Magalhães, J.M.C.S.; Moreira, M.; Guido, L.; Gomes, A.M.; Delerue-Matos, C. Response surface evaluation of microwave-assisted extraction conditions for Lycium barbarum bioactive compounds. Innov. Food Sci. Emerg. Technol. 2016, 33, 319–326. [Google Scholar] [CrossRef]
- Herrero, M.; Plaza, M.; Cifuentes, A.; Ibáñez, E. Extraction techniques for the determination of phenolic compounds in food. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Academic Press: Oxford, UK, 2012; pp. 159–180. ISBN 978-0-12-381373-2. [Google Scholar]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Azwanida, N.N. A review on the extraction methods use in medicinal plants, principle, strength and limitation. Med. Aromat. Plants 2015, 4, 1–6. [Google Scholar] [CrossRef]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Azmir, J.; Zaidul, I.S.M.; Rahman, M.M.; Sharif, K.M.; Mohamed, A.; Sahena, F.; Jahurul, M.H.A.; Ghafoor, K.; Norulaini, N.A.N.; Omar, A.K.M. Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng. 2013, 117, 426–436. [Google Scholar] [CrossRef]
- Cho, Y.J.; Hong, J.Y.; Chun, H.S.; Lee, S.K.; Min, H.Y. Ultrasonication-assisted extraction of resveratrol from grapes. J. Food Eng. 2006, 77, 725–730. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Da Porto, C.; Porretto, E.; Decorti, D. Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrason. Sonochem. 2013, 20, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Rajha, H.N.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. Effect of alternative physical pretreatments (pulsed electric field, high voltage electrical discharges and ultrasound) on the dead-end ultrafiltration of vine-shoot extracts. Sep. Purif. Technol. 2015, 146, 243–251. [Google Scholar] [CrossRef]
- Ghafoor, K.; Park, J.; Choi, Y.H. Optimization of supercritical fluid extraction of bioactive compounds from grape (Vitis labrusca B.) peel by using response surface methodology. Innov. Food Sci. Emerg. Technol. 2010, 11, 485–490. [Google Scholar] [CrossRef]
- Topal, U.; Sasaki, M.; Goto, M.; Hayakawa, K. Extraction of lycopene from tomato skin with supercritical carbon dioxide: Effect of operating conditions and solubility analysis. J. Agric. Food Chem. 2006, 54, 5604–5610. [Google Scholar] [CrossRef] [PubMed]
- Mira, B.; Blasco, M.; Berna, A.; Subirats, S. Supercritical CO2 extraction of essential oil from orange peel. Effect of operation conditions on the extract composition. J. Supercrit. Fluids 1999, 14, 95–104. [Google Scholar] [CrossRef]
- Dechow, F.J. Purification Techniques in Biotechnology, 3rd ed.; Noyes Publications: Park Ridge, IL, USA, 1989; ISBN 0815511973. [Google Scholar]
- Strathmann, H. Membrane separation processes: Current relevance and future opportunities. AIChe J. 2001, 47, 1077–1087. [Google Scholar] [CrossRef]
- Li, J.; Chase, H.A. Applications of membrane techniques for purification of natural products. Biotechnol. Lett. 2010, 32, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Van der Bruggen, B.; Vandecasteele, C.; Van Gestel, T.; Doyen, W.; Leysen, R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Moura Bernardes, A. General aspects of membrane separation processes. In Electrodyalisis and Water Reuse: Novel Approaches; Moura Bernardes, A., Siqueira Rodrigues, M.A., Zoppas Ferreira, J., Eds.; Springer: London, UK, 2014; pp. 3–10. ISBN 978-3-642-40249-4. [Google Scholar]
- Galanakis, C.M. Separation of functional macromolecules and micromolecules: From ultrafiltration to the border to nanofiltration. Trends Food Sci. Technol. 2015, 42, 44–63. [Google Scholar] [CrossRef]
- Crespo, J.G.; Brazinha, C. Membrane processing: Natural antioxidants from winemaking by-products. Filtr. Sep. 2010, 47, 32–35. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Giorno, L.; Drioli, E. Fractionation of olive mill wastewaters by membrane separation techniques. J. Hazard. Mater. 2013, 248–249, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Turano, E.; Curcio, S.; De Paola, M.G.; Calabrò, V.; Iorio, G. An integrated centrifugation-ultrafiltration system in the treatment of olive mill wastewater. J. Membr. Sci. 2002, 209, 519–531. [Google Scholar] [CrossRef]
- Paraskeva, C.A.; Papadakis, V.G.; Tsarouchi, E.; Kanellopoulou, D.G.; Koutsoukos, P.G. Membrane processing for olive mill wastewater fractionation. Desalination 2007, 213, 218–229. [Google Scholar] [CrossRef]
- Garcia-Castello, E.; Cassano, A.; Criscuoli, A.; Conidi, C.; Drioli, E. Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res. 2010, 44, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Bazzarelli, F.; Piacentini, E.; Poerio, T.; Mazzei, R.; Cassano, A.; Giorno, L. Advances in membrane operations for water purification and biophenols recovery/valorization from OMWWs. J. Membr. Sci. 2016, 497, 402–409. [Google Scholar] [CrossRef]
- Arvaniti, E.C.; Zagklis, D.P.; Papadakis, V.G.; Paraskeva, C.A. High-Added value materials production from OMW: A technical and economical optimization. Int. J. Chem. Eng. 2012, 1–7. [Google Scholar] [CrossRef]
- Romani, A.; Scardigli, A.; Pinelli, P. An environmentally friendly process for the production of extracts rich in phenolic antioxidants from Olea europaea L. and Cynara scolymus L. matrices. Eur. Food Res. Technol. 2017, 243, 1229–1238. [Google Scholar] [CrossRef]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Food 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Garcia-Castello, E. Valorization of artichoke wastewaters by integrated membrane process. Water Res. 2014, 48, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Conidi, C.; Rodriguez-Lopez, A.D.; Garcia-Castello, E.M.; Cassano, A. Purification of artichoke polyphenols by using membrane filtration and polymeric resins. Sep. Purif. Technol. 2015, 144, 153–161. [Google Scholar] [CrossRef]
- Cassano, A.; Cabri, W.; Mombelli, G.; Peterlongo, F.; Giorno, L. Recovery of bioactive compounds from artichoke brines by nanofiltration. Food Bioprod. Process. 2016, 98, 257–265. [Google Scholar] [CrossRef]
- Braddock, R.J. Handbook of Citrus By-Products and Processing Technology; John Wiley & Sons, Inc.: New York, NY, USA, 1999; ISBN 9780471190240. [Google Scholar]
- Simone, S.; Conidi, C.; Ursino, C.; Cassano, A.; Figoli, A. Clarification of orange press liquors by PVDF hollow fiber membranes. Membranes 2016, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Braddock, R.J. Ultrafiltration and reverse osmosis recovery of limonene from citrus processing waste streams. J. Food Sci. 1982, 47, 946–948. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Ruby-Figueroa, R. Recovery of flavonoids from orange press liquor by an integrated membrane process. Membranes 2014, 4, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Di Donna, L.; Iacopetta, D.; Cappello, A.R.; Gallucci, G.; Martello, E.; Fiorillo, M.; Dolce, E.; Sindona, G. Hypocholesterolaemic activity of 3-hydroxy-3-methyl-glutaryl flavanones enriched fraction from bergamot fruit (Citrus bergamia): “In vivo” studies. J. Funct. Food 2014, 7, 558–568. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Drioli, E. A membrane based study for the recovery of polyphenols from bergamot juice. J. Membr. Sci. 2011, 375, 182–190. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A. Recovery of phenolic compounds from bergamot juice by nanofiltration membranes. Desalination Water Treat. 2015, 56, 3510–3518. [Google Scholar] [CrossRef]
- Cassano, A.; Donato, L.; Drioli, E. Ultrafiltration of kiwifruit juice: Operating parameters, juice quality and membrane fouling. J. Food Eng. 2007, 79, 613–621. [Google Scholar] [CrossRef]
- Astudillo-Castro, C.L. Limiting flux and critical transmembrane pressure determination using an exponential model: The effect of concentration factor, temperature, and cross-flow velocity during casein micelle concentration by microfiltration. Ind. Eng. Chem. Res. 2015, 54, 414–425. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Ultra- and nanofiltration of aqueous extracts from distilled fermented grape pomace. J. Food Eng. 2009, 91, 587–593. [Google Scholar] [CrossRef]
- Todisco, S.; Tallarico, P.; Gupta, B.B. Mass transfer and polyphenols retention in the clarification of black tea with ceramic membranes. Innov. Food Sci. Emerg. Technol. 2002, 3, 255–262. [Google Scholar] [CrossRef]
- Giacobbo, A.; Bernardes, A.M.; de Pinho, M.N. Sequential pressure-driven membrane operations to recover and fractionate polyphenols and polysaccharides from second racking wine lees. Sep. Purif. Technol. 2017, 173, 49–54. [Google Scholar] [CrossRef]
- Zirehpour, A.; Rahimpour, A.; Jahanshahi, M. The filtration performance and efficiency of olive mill wastewater treatment by integrated membrane process. Desalination Water Treat. 2015, 53, 1254–1262. [Google Scholar] [CrossRef]
- Cheryan, M. Ultrafiltration and Microfiltration Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1998; ISBN 9781566765985. [Google Scholar]
- Jiraratananon, R.; Chanachai, A. A study of fouling in the ultrafiltration of passion fruit juice. J. Membr. Sci. 1996, 111, 39–48. [Google Scholar] [CrossRef]
- Giacobbo, A.; Oliveira, M.; Duarte, E.C.N.F.; Mira, H.M.C.; Bernardes, A.M.; de Pinho, M.N. Ultrafiltration based process for the recovery of polysaccharides and polyphenols from winery effluents. Sep. Sci. Technol. 2013, 48, 438–444. [Google Scholar] [CrossRef]
- Patel, T.M.; Nath, K. Modeling of permeate flux and mass transfer resistances in the reclamation of molasses wastewater by a novel gas-sparged nanofiltration. Korean J. Chem. Eng. 2014, 31, 1865–1876. [Google Scholar] [CrossRef]
- Tsuru, T.; Sudoh, T.; Yoshioka, T.; Asaeda, M. Nanofiltration in non-aqueous solutions by porous silica-zirconia membranes. J. Membr. Sci. 2001, 185, 253–261. [Google Scholar] [CrossRef]
- Bordenave, N.; Bruce, R.; Hamaker, B.R.; Ferruzzi, M.G. Nature and consequences of non-covalent interactions between flavonoids and macronutrients in foods. Food Funct. 2014, 5, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Conidi, C.; Cassano, A.; Drioli, E. Recovery of phenolic compounds from orange press liquor by nanofiltration. Food Bioprod. Process. 2012, 90, 867–874. [Google Scholar] [CrossRef]
- Tylkowski, B.; Tsibranska, I.; Kochanova, R.; Peeva, G.; Marta, G. Concentration of biologically active compounds extracted from Sideritis ssp. L. by nanofiltration. Food Bioprod. Process. 2011, 89, 307–314. [Google Scholar] [CrossRef]
- Pinto, P.C.R.; Mota, I.F.; Loureiro, J.M.; Rodrigues, A.E. Membrane performance and application of ultrafiltration and nanofiltration to ethanol/water extract of Eucalyptus bark. Sep. Purif. Technol. 2014, 132, 234–243. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Caiazzo, F.; Drioli, E. Separation and purification of phenolic compounds from pomegranate juice by ultrafiltration and nanofiltration membranes. J. Food Eng. 2017, 195, 1–13. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Markouli, E.; Gekas, V. Recovery and fractionation of different phenolic classes from winery sludge using ultrafiltration. Sep. Purif. Technol. 2013, 107, 245–251. [Google Scholar] [CrossRef]
- Liu, D.; Vorobiev, E.; Savoire, R.; Lanoisellé, J.L. Intensification of polyphenols extraction from grape seeds by high voltage electrical discharges and extract concentration by dead-end ultrafiltration. Sep. Purif. Technol. 2011, 81, 134–140. [Google Scholar] [CrossRef]
- Benítez, F.J.; Acero, J.L.; Leal, A.I.; González, M. The use of ultrafiltration and nanofiltration membranes for the purification of cork processing wastewater. J. Hazard. Mater. 2009, 162, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Kalbasi, A.; Cisneros-Zevallos, L. Fractionation of monomeric and polymeric anthocyanins from Concord grape (Vitis labrusca L.) juice by membrane ultrafiltration. J. Agric. Food Chem. 2007, 55, 7036–7042. [Google Scholar] [CrossRef] [PubMed]
- Russo, C. A new membrane process for the selective fractionation and total recovery of polyphenols, water and organic substances from vegetation waters (VW). J. Membr. Sci. 2007, 288, 239–246. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Drioli, E. Comparison of the performance of UF membranes in olive mill wastewaters treatment. Water Res. 2011, 45, 3197–3204. [Google Scholar] [CrossRef] [PubMed]
- Susanto, H.; Feng, Y.; Ulbricht, M. Fouling behavior of aqueous solutions of polyphenolic compounds during ultrafiltration. J. Food Eng. 2009, 91, 333–340. [Google Scholar] [CrossRef]
- Boussu, K.; Vandecasteele, C.; Van der Bruggen, B. Relation between membrane characteristics and performance in nanofiltration. J. Membr. Sci. 2008, 310, 51–65. [Google Scholar] [CrossRef]
- Sotto, A.; Arsuaga, J.M.; Van der Bruggen, B. Sorption of phenolic compounds on NF/RO membrane surfaces: Influence on membrane performance. Desalination 2013, 309, 64–73. [Google Scholar] [CrossRef]
- Arsuaga, J.M.; López-Muñoz, M.J.; Sotto, A. Correlation between retention and adsorption of phenolic compounds in nanofiltration membranes. Desalination 2010, 250, 829–832. [Google Scholar] [CrossRef]
- Arsuaga, J.M.; Sotto, A.; López-Muñoz, M.J.; Braeken, L. Influence of type and position of functional groups of phenolic compounds on NF/RO performance. J. Membr. Sci. 2011, 372, 380–386. [Google Scholar] [CrossRef]
- Machado, M.T.C.; Mello, B.C.B.S.; Hubinger, M.D. Study of alcoholic and aqueous extraction of pequi (Caryocar brasiliense Camb.) natural antioxidants and extracts concentration by nanofiltration. J. Food Eng. 2013, 117, 450–457. [Google Scholar] [CrossRef]








| Membrane Process | Required Pressure (Bar) | Typical Separation Mechanism | |
|---|---|---|---|
| Min. | Max. | ||
| Microfiltration | 0.1 | 2 | Sieving |
| Ultrafiltration | 0.1 | 7 | Sieving |
| Nanofiltration | 3 | 25 | Sieving & charge effect |
| pH | 5.0 |
| Total soluble solids (g/kg) | 29.0 |
| Total suspended solids (%, w/w) | 3.8 |
| Total organic carbon (mg/L) | 13,436 |
| Total inorganic carbon (mg/L) | 10.0 |
| Total phenols (mg/L gallic acid) | 1409 |
| Hydroxyl-tyrosol (mg/L) | 3.8 |
| Protocatechuic acid (mg/L) | 25.0 |
| Catechol (mg/L) | 7.5 |
| Tyrosol (mg/L) | 39.0 |
| Caffeic acid (mg/L) | 5.0 |
| p-cumaric acid (mg/L) | 1.0 |
| Total Suspended Solids (%, w/w) | 2.5 ± 0.1 |
| Total soluble solids (g/kg) | 30.5 ± 0.5 |
| Glucose (mg/L) | 960.0 ± 1.0 |
| Fructose (mg/L) | 837.0 ± 1.1 |
| Sucrose (mg/L) | 1050.0 ± 0.4 |
| TAA (mM Trolox) | 8.00 ± 0.04 |
| Chlorogenic acid (mg/L) | 251.0 ± 2.6 |
| Cynarin (mg/L) | 164.7 ± 1.41 |
| Apigenin-7-O-glucoside (mg/L) | 101.0 ± 2.0 |
| Suspended Solids (%) | 8.3 ± 0.2 |
| TSS (°Brix) | 18.0 ± 0.1 |
| pH | 3.5 ± 0.2 |
| TAA (mM Trolox) | 21.4 ± 3.5 |
| Total polyphenols (as GAE) (ppm) | 1217.3 ± 57.0 |
| Neohesperidin (ppm) | 20.7 ± 0.4 |
| Hesperidin (ppm) | 18.4 ± 0.3 |
| Naringin (ppm) | 5.5 ± 0.1 |
| Analysed Compounds | Membrane Type | |||
|---|---|---|---|---|
| Etna 01 PP (1000 Da) | PES004 (4000 Da) | MPF-36 (1000 Da) | Desal GK (2000 Da) | |
| Glucose | 3.31 ± 4.96 | 4.18 ± 0.46 | 3.86 ± 1.20 | 1.61 ± 0.01 |
| Fructose | 2.36 ± 0.04 | 3.17 ± 0.84 | 9.92 ± 1.11 | 2.17 ± 0.84 |
| Total polyphenols | 85.22 ± 0.23 | 94.97 ± 0.01 | 97.50 ± 0.02 | 88.25 ± 0.34 |
| TAA | 57.11 ± 2.72 | 85.61 ± 0.87 | 95.23 ± 0.90 | 78.15 ± 3.72 |
| Cyanidin 3,5-O-diglucoside | 72.52 ± 0.45 | 98.54 ± 0.48 | 99.54 ± 0.13 | 92.77 ± 0.48 |
| Cyanidin 3-O-glucoside | 67.52 ± 1.58 | 90.44 ± 0.81 | 98.88 ± 0.13 | 82.17 ± 0.08 |
| Delphinidin 3-O-glucoside | 69.95 ± 1.53 | 93.99 ± 3.40 | 98.94 ± 0.39 | 83.60 ± 0.46 |
| Pelargolidin 3,5-O-diglucoside | 84.11 ± 2.52 | 63.13 ± 0.23 | 80.42 ± 4.95 | 79.90 ± 0.81 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cassano, A.; Conidi, C.; Ruby-Figueroa, R.; Castro-Muñoz, R. Nanofiltration and Tight Ultrafiltration Membranes for the Recovery of Polyphenols from Agro-Food By-Products. Int. J. Mol. Sci. 2018, 19, 351. https://doi.org/10.3390/ijms19020351
Cassano A, Conidi C, Ruby-Figueroa R, Castro-Muñoz R. Nanofiltration and Tight Ultrafiltration Membranes for the Recovery of Polyphenols from Agro-Food By-Products. International Journal of Molecular Sciences. 2018; 19(2):351. https://doi.org/10.3390/ijms19020351
Chicago/Turabian StyleCassano, Alfredo, Carmela Conidi, René Ruby-Figueroa, and Roberto Castro-Muñoz. 2018. "Nanofiltration and Tight Ultrafiltration Membranes for the Recovery of Polyphenols from Agro-Food By-Products" International Journal of Molecular Sciences 19, no. 2: 351. https://doi.org/10.3390/ijms19020351
APA StyleCassano, A., Conidi, C., Ruby-Figueroa, R., & Castro-Muñoz, R. (2018). Nanofiltration and Tight Ultrafiltration Membranes for the Recovery of Polyphenols from Agro-Food By-Products. International Journal of Molecular Sciences, 19(2), 351. https://doi.org/10.3390/ijms19020351