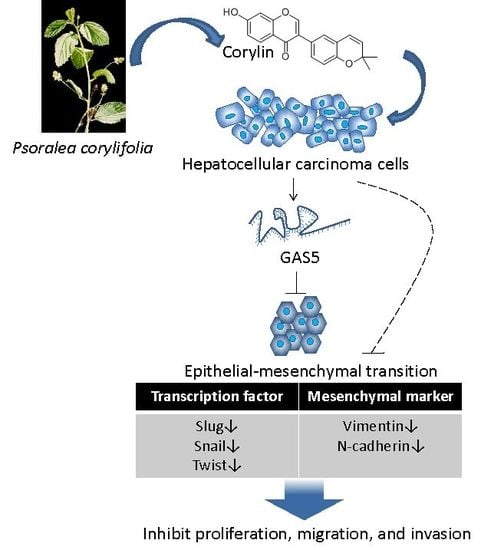
Corylin Suppresses Hepatocellular Carcinoma Progression via the Inhibition of Epithelial-Mesenchymal Transition, Mediated by Long Noncoding RNA GAS5
Abstract
:1. Introduction
2. Results
2.1. Corylin Inhibits the Proliferation, Migration, and Invasiveness of HCC Cells
2.2. Corylin Inhibits Epithelial-Mesenchymal Transition (EMT)
2.3. Corylin Inhibits Tumor Growth in Mice
2.4. Corylin Can Inhibit the Activation of Signaling Pathways Associated with Cell Growth and Apoptosis
2.5. Corylin Exerts Its Anticancer Effects by Inducing lncRNA GAS5
3. Discussion
4. Materials and Methods
4.1. Cell Lines, Antibodies, Drug, and siRNA
4.2. Cell Proliferation Assay
4.3. Cell Migration and Invasion Assays
4.4. Whole-Transcriptome Sequencing
4.5. Detection of lncRNA GAS5 Levels Using Quantitative Real-Time RT-PCR
4.6. Transfection and Western Blotting
4.7. Xenograft Assays and Drug Administration
4.8. Immunohistochemistry
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| TCM | Traditional Chinese medicine |
| HPLC | High-performance liquid chromatography |
| CE | Capillary electrophoresis |
| GC | Gas chromatography |
| TLC | Thin-layer chromatography |
| lncRNA | Long noncoding RNA |
| EMT | Epithelial–mesenchymal transition |
| IC50 | Half-maximal inhibitory concentration |
| DMSO | Dimethyl sulfoxide |
References
- Wang, B.; Chou, Y.E.; Lien, M.Y.; Su, C.M.; Yang, S.F.; Tang, C.H. Impacts of CCL4 gene polymorphisms on hepatocellular carcinoma susceptibility and development. Int. J. Med. Sci. 2017, 14, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.X.; Seto, W.K.; Lai, C.L.; Yuen, M.F. Epidemiology of Hepatocellular Carcinoma in the Asia-Pacific Region. Gut Liver 2016, 10, 332–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llovet, J.M. Liver cancer: Time to evolve trial design after everolimus failure. Nat. Rev. Clin. Oncol. 2014, 11, 506–507. [Google Scholar] [CrossRef] [PubMed]
- Worns, M.A.; Galle, P.R. HCC therapies—Lessons learned. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Le Grazie, M.; Biagini, M.R.; Tarocchi, M.; Polvani, S.; Galli, A. Chemotherapy for hepatocellular carcinoma: The present and the future. World J. Hepatol. 2017, 9, 907–920. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Liu, H.; Ming, L. Multiple Roles of Autophagy in the Sorafenib Resistance of Hepatocellular Carcinoma. Cell. Physiol. Biochem. 2017, 44, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Liu, L.; Yang, S.; Ren, J.; Lai, P.B.S.; Chen, G.G. New insights into sorafenib resistance in hepatocellular carcinoma: Responsible mechanisms and promising strategies. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J.; Caballero, R.; Delpon, E. Cancer chemotherapy and cardiac arrhythmias: A review. Drug Saf. 2015, 38, 129–152. [Google Scholar] [CrossRef] [PubMed]
- Lees, J.G.; Makker, P.G.; Tonkin, R.S.; Abdulla, M.; Park, S.B.; Goldstein, D.; Moalem-Taylor, G. Immune-mediated processes implicated in chemotherapy-induced peripheral neuropathy. Eur. J. Cancer 2017, 73, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Le, D.L.; Cao, H.; Yang, L.X. Cardiotoxicity of molecular-targeted drug therapy. Anticancer Res. 2014, 34, 3243–3249. [Google Scholar] [PubMed]
- Ma, X.; Li, R.S.; Wang, J.; Huang, Y.Q.; Li, P.Y.; Wang, J.; Su, H.B.; Wang, R.L.; Zhang, Y.M.; Liu, H.H.; et al. The Therapeutic Efficacy and Safety of Compound Kushen Injection Combined with Transarterial Chemoembolization in Unresectable Hepatocellular Carcinoma: An Update Systematic Review and Meta-Analysis. Front. Pharmacol. 2016, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Luk, J.M.; Wang, X.; Liu, P.; Wong, K.F.; Chan, K.L.; Tong, Y.; Hui, C.K.; Lau, G.K.; Fan, S.T. Traditional Chinese herbal medicines for treatment of liver fibrosis and cancer: From laboratory discovery to clinical evaluation. Liver Int. 2007, 27, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; An, H.M.; Wang, S.S.; Chen, J.J.; Xu, L. Preventive and Therapeutic Effects of Chinese Herbal Compounds against Hepatocellular Carcinoma. Molecules 2016, 21, 142. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, J.X.; Li, H.; Baak, J.P.A. In Metastatic Non-small cell Lung Cancer Platinum-Based Treated Patients, Herbal Treatment Improves the Quality of Life. A Prospective Randomized Controlled Clinical Trial. Front. Pharmacol. 2017, 8, 454. [Google Scholar] [CrossRef] [PubMed]
- Li-Weber, M. Targeting apoptosis pathways in cancer by Chinese medicine. Cancer Lett. 2013, 332, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Bent, S. Herbal medicine in the United States: Review of efficacy, safety, and regulation: Grand rounds at University of California, San Francisco Medical Center. J. Gen. Intern. Med. 2008, 23, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zeng, Y.; Wan, M.; Li, R.; Liang, Y.; Li, C.; Zeng, Z.; Chau, F.T. Comparative analysis of essential oils from eight herbal medicines with pungent flavor and cool nature by GC-MS and chemometric resolution methods. J. Sep. Sci. 2009, 32, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Lacikova, L.; Jancova, M.; Muselik, J.; Masterova, I.; Grancai, D.; Fickova, M. Antiproliferative, cytotoxic, antioxidant activity and polyphenols contents in leaves of four Staphylea L. species. Molecules 2009, 14, 3259–3267. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zou, H.; Kong, L.; Ni, J. Analysis of bioactive components in traditional Chinese medicines by molecular biochromatography with α-acid glycoprotein stationary phase. J. Basic Clin. Physiol. Pharmacol. 2000, 11, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cai, T.; Xia, X.; Cai, Y.; Wu, X.Y. Research Advances in the Intervention of Inflammation and Cancer by Active Ingredients of Traditional Chinese Medicine. J. Pharm. Pharm. Sci. 2016, 19, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Houh, Y.K.; Kim, K.E.; Park, S.; Hur, D.Y.; Kim, S.; Kim, D.; Bang, S.I.; Yang, Y.; Park, H.J.; Cho, D. The Effects of Artemisinin on the Cytolytic Activity of Natural Killer (NK) Cells. Int. J. Mol. Sci. 2017, 18, 1600. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.X.; Li, Y.; Yin, H.; Zhang, J. Curcumin: Updated molecular mechanisms and intervention targets in human lung cancer. Int. J. Mol. Sci. 2012, 13, 3959–3978. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Tan, H.Y.; Li, S.; Cheung, F.; Wang, N.; Nagamatsu, T.; Feng, Y. Cancer Stem Cells: The Potential Targets of Chinese Medicines and Their Active Compounds. Int. J. Mol. Sci. 2016, 17, 893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terlikowska, K.M.; Witkowska, A.M.; Zujko, M.E.; Dobrzycka, B.; Terlikowski, S.J. Potential application of curcumin and its analogues in the treatment strategy of patients with primary epithelial ovarian cancer. Int. J. Mol. Sci. 2014, 15, 21703–21722. [Google Scholar] [CrossRef] [PubMed]
- Slezakova, S.; Ruda-Kucerova, J. Anticancer Activity of Artemisinin and its Derivatives. Anticancer Res. 2017, 37, 5995–6003. [Google Scholar] [PubMed]
- Qin, G.; Zhao, C.; Zhang, L.; Liu, H.; Quan, Y.; Chai, L.; Wu, S.; Wang, X.; Chen, T. Dihydroartemisinin induces apoptosis preferentially via a Bim-mediated intrinsic pathway in hepatocarcinoma cells. Apoptosis 2015, 20, 1072–1086. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Z.; Li, L.; Liu, M.Y.; Jin, X.B.; Mao, J.W.; Pu, Q.H.; Meng, M.J.; Chen, X.G.; Zhu, J.Y. Curcumin induces FasL-related apoptosis through p38 activation in human hepatocellular carcinoma Huh7 cells. Life Sci. 2013, 92, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wang, Y.; He, X.; Zhang, Z.; Yin, Q.; Chen, Y.; Yu, H.; Huang, Y.; Chen, L.; Xu, M.; et al. Codelivery of sorafenib and curcumin by directed self-assembled nanoparticles enhances therapeutic effect on hepatocellular carcinoma. Mol. Pharm. 2015, 12, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Zuo, M.; Li, C.; Lin, J.; Javle, M. LLL12, a novel small inhibitor targeting STAT3 for hepatocellular carcinoma therapy. Oncotarget 2015, 6, 10940–10949. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Wang, T.L.; Zhang, Z.Q.; Yang, W.Q.; Wang, Y.F.; Chai, X.; Wang, C.H.; Li, Z. Phytochemical and Pharmacological Studies on the Genus Psoralea: A Mini Review. Evid. Based Complement. Altern. Med. 2016, 2016, 8108643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, W.; Wang, Y.; Lu, J.; Chen, X. The Chemical Constituents and Bioactivities of Psoralea corylifolia Linn.: A Review. Am. J. Chin. Med. 2016, 44, 35–60. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.L.; Fang, S.H.; Wang, S.C.; Cheng, W.C.; Liu, P.L.; Su, C.C.; Chen, C.S.; Huang, M.Y.; Hua, K.F.; Shen, K.H.; et al. Corylin protects LPS-induced sepsis and attenuates LPS-induced inflammatory response. Sci. Rep. 2017, 7, 46299. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lim, H.S.; Lee, J.; Jeong, S.J. Quantitative Analysis of Psoralea corylifolia Linne and its Neuroprotective and Anti-Neuroinflammatory Effects in HT22 Hippocampal Cells and BV-2 Microglia. Molecules 2016, 21, 1076. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Wang, D.; Xu, Y.; Li, F. Osteoblastic differentiation bioassay and its application to investigating the activity of fractions and compounds from Psoralea corylifolia L. Pharmazie 2003, 58, 925–928. [Google Scholar] [PubMed]
- Wang, D.; Li, F.; Jiang, Z. Osteoblastic proliferation stimulating activity of Psoralea corylifolia extracts and two of its flavonoids. Planta Med. 2001, 67, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Yun, B.R.; Kim, M.H.; Park, C.S.; Lee, W.S.; Oh, H.M.; Rho, M.C. Phenolic compounds isolated from Psoralea corylifolia inhibit IL-6-induced STAT3 activation. Planta Med. 2012, 78, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Giroud, M.; Scheideler, M. Long Non-Coding RNAs in Metabolic Organs and Energy Homeostasis. Int. J. Mol. Sci. 2017, 18, 2578. [Google Scholar] [CrossRef] [PubMed]
- Cech, T.R.; Steitz, J.A. The noncoding RNA revolution-trashing old rules to forge new ones. Cell 2014, 157, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, A.M.; Chang, H.Y. Long Noncoding RNAs in Cancer Pathways. Cancer Cell 2016, 29, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Khanduja, J.S.; Calvo, I.A.; Joh, R.I.; Hill, I.T.; Motamedi, M. Nuclear Noncoding RNAs and Genome Stability. Mol. Cell 2016, 63, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yang, Y.; Xu, C.; Guo, J. Regulatory mechanisms of long noncoding RNAs on gene expression in cancers. Cancer Genet. 2017, 216–217, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Bartonicek, N.; Maag, J.L.; Dinger, M.E. Long noncoding RNAs in cancer: Mechanisms of action and technological advancements. Mol. Cancer 2016, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.H.; Chan, C.W.; Fang, J.Y.; Shih, Y.M.; Liu, Y.W.; Wang, T.V.; Chen, C.Y. 2-O-Methylmagnolol upregulates the long non-coding RNA, GAS5, and enhances apoptosis in skin cancer cells. Cell Death Dis. 2017, 8, e2638. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yan, I.K.; Wood, J.; Haga, H.; Patel, T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol. Cancer Res. 2014, 12, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Li, C.; Lan, T.; Wu, L.; Yuan, Y.; Liu, Q.; Liu, Z. Decreased expression of long non-coding RNA GAS5 indicates a poor prognosis and promotes cell proliferation and invasion in hepatocellular carcinoma by regulating vimentin. Mol. Med. Rep. 2016, 13, 1541–1550. [Google Scholar] [CrossRef] [PubMed]
- Mourtada-Maarabouni, M.; Pickard, M.R.; Hedge, V.L.; Farzaneh, F.; Williams, G.T. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene 2009, 28, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Pickard, M.R.; Mourtada-Maarabouni, M.; Williams, G.T. Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochim. Biophys. Acta Mol. Basis Dis. 2013, 1832, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Mazar, J.; Rosado, A.; Shelley, J.; Marchica, J.; Westmoreland, T.J. The long non-coding RNA GAS5 differentially regulates cell cycle arrest and apoptosis through activation of BRCA1 and p53 in human neuroblastoma. Oncotarget 2017, 8, 6589–6607. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.Q.; Li, R.J.; Mei, J.Z.; Li, X.H. Down-regulation of long non-coding RNA GAS5 is associated with the prognosis of hepatocellular carcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 4303–4309. [Google Scholar] [PubMed]
- Wang, T.H.; Lin, Y.S.; Chen, Y.; Yeh, C.T.; Huang, Y.L.; Hsieh, T.H.; Shieh, T.M.; Hsueh, C.; Chen, T.C. Long non-coding RNA AOC4P suppresses hepatocellular carcinoma metastasis by enhancing vimentin degradation and inhibiting epithelial-mesenchymal transition. Oncotarget 2015, 6, 23342–23357. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-Y.; Chen, C.-C.; Shieh, T.-M.; Hsueh, C.; Wang, S.-H.; Leu, Y.-L.; Lian, J.-H.; Wang, T.-H. Corylin Suppresses Hepatocellular Carcinoma Progression via the Inhibition of Epithelial-Mesenchymal Transition, Mediated by Long Noncoding RNA GAS5. Int. J. Mol. Sci. 2018, 19, 380. https://doi.org/10.3390/ijms19020380
Chen C-Y, Chen C-C, Shieh T-M, Hsueh C, Wang S-H, Leu Y-L, Lian J-H, Wang T-H. Corylin Suppresses Hepatocellular Carcinoma Progression via the Inhibition of Epithelial-Mesenchymal Transition, Mediated by Long Noncoding RNA GAS5. International Journal of Molecular Sciences. 2018; 19(2):380. https://doi.org/10.3390/ijms19020380
Chicago/Turabian StyleChen, Chi-Yuan, Chin-Chuan Chen, Tzong-Ming Shieh, Chuen Hsueh, Shu-Huei Wang, Yann-Lii Leu, Jang-Hau Lian, and Tong-Hong Wang. 2018. "Corylin Suppresses Hepatocellular Carcinoma Progression via the Inhibition of Epithelial-Mesenchymal Transition, Mediated by Long Noncoding RNA GAS5" International Journal of Molecular Sciences 19, no. 2: 380. https://doi.org/10.3390/ijms19020380
APA StyleChen, C. -Y., Chen, C. -C., Shieh, T. -M., Hsueh, C., Wang, S. -H., Leu, Y. -L., Lian, J. -H., & Wang, T. -H. (2018). Corylin Suppresses Hepatocellular Carcinoma Progression via the Inhibition of Epithelial-Mesenchymal Transition, Mediated by Long Noncoding RNA GAS5. International Journal of Molecular Sciences, 19(2), 380. https://doi.org/10.3390/ijms19020380