Antioxidative and Anti-Melanogenic Activities of Bamboo Stems (Phyllostachys nigra variety henosis) via PKA/CREB-Mediated MITF Downregulation in B16F10 Melanoma Cells
Abstract
:1. Introduction
2. Results
2.1. Bamboo Stem Extraction and Antioxidative Activities of the Extract
2.2. Chemical Composition of the EtOAc fraction of 80% EtOH extract (PN3)
2.3. Inhibition of Fenton’s Reaction
2.4. Inhibition of In Vitro Tyrosinase Activity and Melanin Synthesis
2.5. PN3 Downregulated Melanin Synthesis Genes
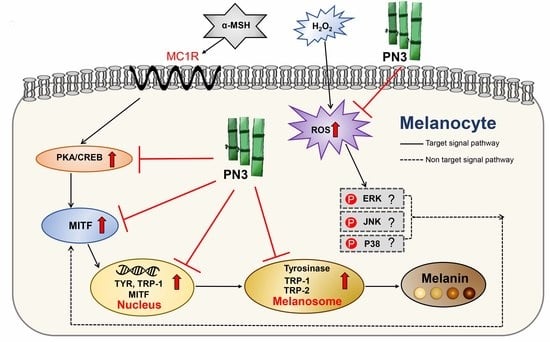
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Bamboo Stem Extracts
4.3. Antioxidative Activity Assay
4.3.1. DPPH Radical Scavenging Activity
4.3.2. ABTS Radical Scavenging Activity
4.3.3. Hydroxyl Radical Scavenging Activity
4.4. Total Phenol and Flavonoid Contents
4.5. High-Performance Liquid Chromatography with Diode-Array Detection (HPLC–DAD) Analysis
4.6. LC–Q–TOF-MS Analysis
4.7. Tyrosinase Inhibition Assay—A Cell-Free System
4.8. Cell Culture
4.9. MTT Cell Viability Assay
4.10. Measurement of Melanin Content
4.11. Immunoblot Analysis
4.12. Fenton Reaction Inhibition Assay
4.13. RNA Isolation and RT-PCR
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| AKT | protein kinase B |
| α-MSH | α-Melanocyte-stimulating hormone |
| cAMP | cyclic adenosine monophosphate |
| COA | p-coumaric acid |
| COAE | p-coumaric acid equivalent |
| CREB | cAMP response element-binding protein |
| DCFH-DA | dichloro-dihydro-fluorescein diacetate |
| DHICA | 5,6-dihydroxyindole-2-carboxylic acid |
| DMSO | dimethyl sulfoxide |
| DOPA | 3,4-dihydroxyphenylalanine |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EtOAc | ethyl acetate |
| EtOH | ethanol |
| GSK-3 | glycogen synthase kinase 3 |
| HPLC | high-performance liquid chromatography |
| IC50 | inhibitory concentration 50 |
| LC–Q–TOF–MS | liquid chromatography–quadropole–time of flight-mass spectrometry |
| MAPKs | mitogen-activated protein kinase |
| MC1R | melanocortin 1 receptor |
| MeOH | methanol |
| MITF | microphthalmia-associated transcription factor |
| MS | mass spectrometry |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| ORAC | oxygen radical absorbance capacity |
| p-CREB | phosphorylation of cAMP response element-binding protein |
| p-PKA | phosphorylation of protein kinase A |
| PKA | protein kinase A |
| PI3K | phosphatidylinositol-4,5-bisphosphate 3-kinase |
| PN3 | ethylacetate fraction of 80% ethanol extracts from the leaves of Phyllostachys nigra var. henonis |
| Q | quercetin |
| QE | quercetin equivalent |
| ROS | reactive oxygen species |
| RT | retention time; bromide |
| RT-PCR | reverse transcription polymerase chain reaction |
| SOD | superoxide dismutase |
| TE | trolox equivalent |
| TRP-1 | Tyrosinase-related protein 1 |
| TRP-2 | Tyrosinase-related protein 2 |
| TYR | Tyrosinase |
References
- Nayak, M.; Nagarajan, A.; Majeed, M.; Mundkur, L.A. Evaluation of in vitro antioxidant potential, anti-inflammatory activity and melanogenesis inhibition of Artocarpus hirsutus Lam. extracts. IJSTR 2017, 6, 196–203. [Google Scholar]
- Park, E.J.; Jhon, D.Y. Effects of bamboo shoot consumption on lipid profiles and bowel function in healthy young women. Nutrition 2009, 25, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Xia, D.; Huang, W.; Wu, X.; Zhang, Y.; Yao, Y. Hypolipidemic effect of bamboo shoot oil (P. pubescens) in Sprague–Dawley rats. J. Food Sci. 2010, 75, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, Y.; Kimura, R.; Kato, N.; Fujii, T.; Seki, M.; Endo, T.; Kawashima, K. Evolutional study on acetylcholine expression. Life Sci. 2003, 72, 1745–1756. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, W.J.; Chang, S.E.; Lee, G.Y. Hesperidin, a popular antioxidant inhibits melanogenesis via PKA/CREBmediated MITF degradation. Int. J. Mol. Sci. 2015, 16, 18384–18395. [Google Scholar] [CrossRef] [PubMed]
- Yoon, N.Y.; Eom, T.K.; Kim, M.M.; Kim, S.K. Inhibitory effect of phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J. Agric. Food Chem. 2009, 57, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- An, S.M.; Lee, S.I.; Choi, S.W.; Moon, S.W.; Boo, Y.C. p-Coumaric acid, a constituent of Sasa quelpaertensis Nakai, inhibits cellular melanogenesis stimulated by α-melanocyte stimulating hormone. Br. J. Dermatol. 2008, 159, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Ryu, M.; Suzuki, Y.A. Lactoferrin inhibits melanogenesis by down-regulating MITF in melanoma cells and normal melanocytes. Biochem. Cell Biol. 2017, 95, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Guo, Y.; Zhang, Y.; Zhuang, Y. Antioxidant and anti-tyrosinase activities of phenolic extracts from rape bee pollen and inhibitory melanogenesis by cAMP/MITF/TYR pathway in B16 mouse melanoma cells. Front. Pharm. 2017, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V.J. Determination of melanin synthetic pathways. J. Investig. Dermatol. 2011, 17, E8–E11. [Google Scholar] [CrossRef] [PubMed]
- Rho, S.; Chung, H.S.; Kang, M.; Lee, E.; Cho, C.; Kim, H.; Park, S.; Kim, H.Y.; Hong, M.; Shin, M.; et al. Inhibition of production of reactive oxygen species and gene expression profile by treatment of ethanol extract of Moutan Cortex Radicis in oxidative stressed PC12 cells. Biol. Pharm. Bull. 2005, 28, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Jun, M.; Tohru, U.; Li, J.Z.; Takeshi, F. Identification and evaluation of antioxidant activities of bamboo extracts. For. Sci. Pract. 2004, 6, 1–5. [Google Scholar]
- Lim, J.A.; Na, Y.S.; Baek, S.H. Antioxidative activity and nitrite scavenging ability of ethanol extract from Phyllostachys bambusoides. Korean J. Food Sci. Technol. 2004, 36, 306–310. [Google Scholar]
- Tanaka, A.; Zhu, Q.; Tan, H.; Horiba, H.; Ohnuki, K.; Mori, Y.; Yamauchi, R.; Ishikawa, H.; Iwamoto, A.; Kawahara, H.; et al. Biological activities and phytochemical profiles of extracts from different parts of bamboo (Phyllostachys pubescens). Molecules 2014, 19, 8238–8260. [Google Scholar] [CrossRef] [PubMed]
- Keski-Saari, S.; Ossipov, V.; Julkunen-Tiitto, R.; Jia, J.; Danell, K.; Veteli, T.; Guiquan, Z.; Yaowu, X.; Niemelä, P. Phenolics from the culms of five bamboo species in the Tangjiahe and Wolong Giant Panda Reserves, Sichuan, China. Biochem. Syst. Ecol. 2008, 36, 758–765. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Wu, X.; Lu, B.; Zhang, Y. Safety evaluation of polyphenol-rich extract from bamboo shavings. Afr. J. Biotechnol. 2010, 9, 77–86. [Google Scholar]
- Julkunen-Tiitto, R.; Rousi, M.; Bryant, J.P.; Sorsa, S.; Keinänen, M.; Sikanen, H. Chemical diversity of several Betulaceae species: Comparison of phenolics and terpenoids in northern birch stems. Trees 1996, 11, 16–22. [Google Scholar] [CrossRef]
- Laitinen, J.; Julkunen-Tiitto, R.; Rousi, M.; Heinonen, J.; Tahvanainen, J. Ontogeny and environment as determinants of the secondary chemistry of three species of white birch. J. Chem. Ecol. 2005, 31, 2243–2262. [Google Scholar] [CrossRef] [PubMed]
- Veteli, T.O.; Kuokkanen, K.; Julkunen-Tiitto, R.; Roininen, H.; Tahvanainen, J. Effects of elevated CO2 and temperature on plant growth and herbivore defensive chemistry. Glob. Chang. Biol. 2002, 8, 1240–1252. [Google Scholar] [CrossRef]
- Tegelberg, R.; Veteli, T.; Aphalo, P.J.; Julkunen-Tiitto, R. Clonal differences in growth and phenolics of willows exposed to elevated ultraviolet-B radiation. Basic Appl. Ecol. 2003, 4, 219–228. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Y.; Wu, Y.; Tan, H.; Meng, F.; Wang, Y.S.; Li, M.; Zhao, L.; Liu, L.; Qian, Y.; et al. Analysis of accumulation patterns and preliminary study on the condensation mechanism of proanthocyanidins in the tea plant [Camellia sinensis]. Sci. Rep. 2015, 5, 8742. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, R.; Kuhnert, N. Identification and characterization of five new classes of chlorogenic acids in burdock (Arctium lappa L.) roots by liquid chromatography/tandem mass spectrometry. Food Funct. 2011, 2, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, K.N.; Sudarshana Dissanayake, D.M. Variation of phenolic content in coconut oil extracted by two conventional methods. Int. J. Food Sci. Technol. 2008, 43, 597–602. [Google Scholar] [CrossRef]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in northern chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cao, X.; Jiang, H.; Qi, Y.; Chin, K.L.; Yue, Y. Antioxidant activity of leaf extracts from different Hibiscus sabdariffa accessions and simultaneous determination five major antioxidant compounds by LC-Q-TOF-MS. Molecules 2014, 19, 21226–21238. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.; Arbiser, J.L. The reactive oxygen-driven tumor: Relevance to melanoma. Pigment Cell Melanoma Res. 2008, 21, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Golla, U.; Bhimathati, S.S.R. Evaluation of antioxidant and DNA damage protection activity of the hydroalcoholic extract of Desmostachya bipinnata L. Stapf. Sci. World J. 2014, 2014, 215084. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Park, S.H.; Kwon, S.B.; Li, K.; Youn, S.W.; Park, K.C. (−)-Epigallocatechin-3-gallate and hinokitiol reduce melanin synthesis via decreased MITF production. Arch. Pharm. Res. 2004, 27, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Haycock, J.W.; Rowe, S.J.; Cartledge, S.; Wyatt, A.; Ghanem, G.; Marandini, R.; Rennie, I.G.; MacNeil, S. α-Melanocyte-stimulating hormone reduces impact of proinflammatory cytokine and peroxide-generated oxidative stress on keratinocyte and melanoma cell line. J. Biol. Chem. 2000, 275, 15629–15636. [Google Scholar] [CrossRef] [PubMed]
- Meyskens Jr, F.L.; Farmer, P.; Fruehauf, J.P. Redox regulation in human melanocytes and melanoma. Pigment Cell Melanoma Res. 2001, 14, 148–154. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Jensen, P.E.; Møller, I.M.; Schulz, A. Monitoring reactive oxygen species formation and localisation in living cells by use of the fluorescent probe CM-H2DCFDA and confocal laser microscopy. Physiol. Plant. 2009, 136, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Toriyama, M. Depigmenting effect of catechins. Molecules 2009, 14, 4425–4432. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Jang, J.Y.; Park, C.; Kim, B.W.; Choi, Y.H.; Choi, B.T. Curcumin suppresses α-melanocyte stimulating hormone-stimulated melanogenesis in B16F10 cells. Int. J. Mol. Med. 2010, 26, 101–106. [Google Scholar] [PubMed]
- Yang, Y.M.; Son, Y.O.; Lee, S.A.; Jeon, Y.M.; Lee, J.C. Quercetin inhibits α-MSH-stimulated melanogenesis in B16F10 melanoma cells. Phytother. Res. 2011, 25, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Lee, J.H.; Jeong, D.; Han, I.O.; Oh, E.S. Melanocortin 1 receptor regulates melanoma cell migration by controlling syndecan-2 expression. J. Biol. Chem. 2012, 287, 19326–19335. [Google Scholar] [CrossRef] [PubMed]
- Joyeux, M.; Mortier, F.; Fleurentin, J. Screening of antiradical, antilipoperoxidant and hepatoprotective effects of nine plant extracts used in Caribbean folk medicine. Phytother. Res. 1995, 9, 228–230. [Google Scholar] [CrossRef]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Formation of a thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: The role of superoxide and hydroxyl radicals. FEBS Lett. 1981, 128, 347–352. [Google Scholar] [CrossRef]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Macrini, D.J.; Suffredini, I.B.; Varella, A.D.; Younes, R.N.; Ohara, M.T. Extracts from Amazonian plants have inhibitory activity against tyrosinase: An in vitro evaluation. Brazilian J. Pharm. Sci. 2009, 45, 715–721. [Google Scholar] [CrossRef]
- Teixeira, R.D.S.; Rocha, P.R.; Polonini, H.C.; Brandão, M.A.F.; Chaves, M.D.G.A.M.; Raposo, N.R.B. Mushroom tyrosinase inhibitory activity and major fatty acid constituents of Amazonian native flora oils. Brazilian J. Pharm. Sci. 2012, 48, 399–404. [Google Scholar] [CrossRef]
- Hosoi, J.; Abe, E.; Suda, T.; Kuroki, T. Regulation of melanin synthesis of B16 mouse melanoma cells by 1α, 25-dihydroxyvitamin D3 and retinoic acid. Cancer Res. 1985, 45, 1474–1478. [Google Scholar] [PubMed]
- Yang, J.H.; Choi, M.H.; Yang, S.H.; Cho, S.S.; Park, S.J.; Shin, H.J.; Ki, S.H. Potent anti-Inflammatory and antiadipogenic properties of bamboo (Sasa coreana Nakai) leaves extract and its major constituent flavonoids. J. Agric. Food Chem. 2017, 65, 6665–6673. [Google Scholar] [CrossRef] [PubMed]






| Bamboo Sample | DPPH | ABTS | Hydroxyl Radical |
|---|---|---|---|
| PN1 | 1064.48 ± 107.77 e | 704.99 ± 28.53 c | 698.68 ± 24.40 c,d |
| PN2 | 714.35 ± 39.54 c | 463.81 ± 13.93 b | 560.19 ± 21.85 b,c |
| PN3 | 565.63 ± 17.75 b | 414.61 ± 35.12 b | 509.17 ± 33.76 b |
| PN4 | 2019.67 ± 69.40 f | 669.68 ± 35.62 c | 766.73 ± 22.23 d |
| PN5 | 877.34 ± 63.75 d | 2258.18 ± 125.06 d | 1395.93 ± 222.72 e |
| Positive control | 23.02 ± 0.39 a | 51.86 ± 0.72 a | 90.57 ± 0.60 a |
| Bamboo Sample | Total Polyphenols (COAE mg/g) | Total Flavonoids (QE mg/g) |
|---|---|---|
| PN1 | 0.140 ± 0.015 a | 8.09 ± 0.22 b |
| PN2 | 0.148 ± 0.014 a | 9.24 ± 0.70 c,d |
| PN3 | 0.179 ± 0.022 b | 9.57 ± 0.37 d |
| PN4 | 0.130 ± 0.017 a | 8.64 ± 0.01 b,c |
| PN5 | 0.131 ± 0.016 a | 7.35 ± 0.27 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.-H.; Jo, H.-G.; Yang, J.H.; Ki, S.H.; Shin, H.-J. Antioxidative and Anti-Melanogenic Activities of Bamboo Stems (Phyllostachys nigra variety henosis) via PKA/CREB-Mediated MITF Downregulation in B16F10 Melanoma Cells. Int. J. Mol. Sci. 2018, 19, 409. https://doi.org/10.3390/ijms19020409
Choi M-H, Jo H-G, Yang JH, Ki SH, Shin H-J. Antioxidative and Anti-Melanogenic Activities of Bamboo Stems (Phyllostachys nigra variety henosis) via PKA/CREB-Mediated MITF Downregulation in B16F10 Melanoma Cells. International Journal of Molecular Sciences. 2018; 19(2):409. https://doi.org/10.3390/ijms19020409
Chicago/Turabian StyleChoi, Moon-Hee, Han-Gyo Jo, Ji Hye Yang, Sung Hwan Ki, and Hyun-Jae Shin. 2018. "Antioxidative and Anti-Melanogenic Activities of Bamboo Stems (Phyllostachys nigra variety henosis) via PKA/CREB-Mediated MITF Downregulation in B16F10 Melanoma Cells" International Journal of Molecular Sciences 19, no. 2: 409. https://doi.org/10.3390/ijms19020409
APA StyleChoi, M. -H., Jo, H. -G., Yang, J. H., Ki, S. H., & Shin, H. -J. (2018). Antioxidative and Anti-Melanogenic Activities of Bamboo Stems (Phyllostachys nigra variety henosis) via PKA/CREB-Mediated MITF Downregulation in B16F10 Melanoma Cells. International Journal of Molecular Sciences, 19(2), 409. https://doi.org/10.3390/ijms19020409