Galectin-3: The Impact on the Clinical Management of Patients with Thyroid Nodules and Future Perspectives
Abstract
:1. The Clinical Problem of the Preoperative Characterization of Follicular Thyroid Nodules
2. The Biological Rationale of Galectin-3 Expression in Transformed Thyroid Cells
3. Validation of a Galectin-3 Test Method for Clinical Use
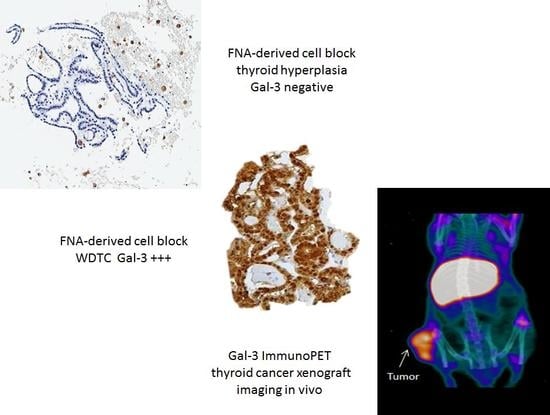
4. Thyroid Cancer Imaging In Vivo by Targeting Galectin-3
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Rosai, J.; Carcangiu, M.L.; De Lellis, R.A. Atlas of Tumor Pathology: Tumors of the Thyroid Gland, 3rd ed.; Armed Force Institute of Pathology: Washington, DC, USA, 1992; pp. 1–343. [Google Scholar]
- Tan, G.H.; Gharib, H. Thyroid incidentalomas: Management approaches to no palpable nodules discovered incidentally on thyroid imaging. Ann. Intern. Med. 1997, 126, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Bartolazzi, A. Improving accuracy of cytology for nodular thyroid lesions. Lancet 2000, 355, 1661–1662. [Google Scholar] [CrossRef]
- Castro, M.R.; Gharib, H. Continuing controversies in the management of thyroid nodules. Ann. Intern. Med. 2005, 142, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, M.J.; Pacini, F. Thyroid nodule. In Thyroid Tumors, 2nd ed.; Editions Nucleon: Paris, France, 2003; pp. 11–31. [Google Scholar]
- Alexander, E.K. Approach to the patient with cytologically indeterminate thyroid nodule. J. Clin. Endocrinol. Metabol. 2008, 93, 4175–4182. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.L.; Gordon, M.; Germann, E.; Robins, R.E.; Mc Gregor, G.I. Clinical parameters predictive of malignancy of thyroid follicular neoplasms. Am. J. Surg. 1991, 161, 567–569. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Lemar, H.; Burch, H.B. Clinical features associated with an increased risk of thyroid malignancy in patients with follicular neoplasia by fine-needle aspiration. Thyroid 1998, 8, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Raber, W.; Kaserer, K.; Niederle, B.; Vierhapper, H. Risk factors for malignancy of thyroid nodules initially identified as follicular neoplasia by fine-needle aspiration: Results of a prospective study of one hundred twenty patients. Thyroid 2000, 10, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Yassa, L.; Cibas, E.S.; Benson, C.B.; Frates, M.C.; Doubilet, P.M.; Gawande, A.A.; Moore, F.D., Jr.; Kim, B.W.; Nosè, W.; Marqusee, E.; et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer 2007, 111, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Rago, T.; Scutari, M.; Latrofa, F.; Loiacono, V.; Piaggi, P.; Marchetti, I.; Romani, R.; Basolo, F.; Miccoli, P.; Tonacchera, M.; et al. The large majority of 1520 patients with indeterminate thyroid nodule at cytology have a favorable outcome and a clinical risk score has a high negative predictive value for a more cumbersome cancer disease. J. Clin. Endocrinol. Metab. 2014, 99, 3700–3707. [Google Scholar] [CrossRef] [PubMed]
- Ianni, F.; Campanella, P.; Rota, C.A.; Prete, A.; Castellino, L.; Pontecorvi, A.; Corsello, S.M. A meta-analysis-derived proposal for a clinical, ultrasonographic and cytological scoring system to evaluate thyroid nodules: The “CUT” score. Endocrine 2016, 52, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Soderstrom, N. Puncture of goiters for aspiration biopsy. Acta Med. Scand. 1952, 144, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Kini, S.R. Thyroid. In Guide to Clinical Aspiration Biopsy; Kline, T.S., Ed.; Igaku Shoin: New York, NY, USA, 1987; Volume 3, pp. 1–380. [Google Scholar]
- Baloch, Z.W.; Sack, M.J.; Yu, G.H.; Li Volsi, V.A.; Gupta, P.K. Fine-needle aspiration of thyroid: An institutional experience. Thyroid 1998, 8, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.L.; Layfield, L.J.; Philippe, A.; Rosenthal, D.L. Sources of diagnostic error in fine needle aspiration of the thyroid. Cancer 1989, 63, 718–725. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Erickson, L.A.; Casey, M.B.; Lam, K.Y.; Lohse, C.M.; Asa, S.L.; Chan, J.K.; De Lellis, R.A.; Harach, H.L.; Kakudo, K.; et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am. J. Surg. Pathol. 2004, 28, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.H.; Jones, J.N.; Qsman, J. Fine-needle aspiration cytology of the thyroid: Ten years experience in a community teaching hospital. Diagn. Cytopathol. 2006, 34, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Schnadig, V.; Logrono, R.; Wasserman, P.G. Fine-needle aspiration of thyroid nodules: A study of 4703 patients with histologic and clinical correlations. Cancer 2007, 111, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Oertel, Y.C.; Miyahara-Felipe, L.; Mendoza, M.G.; Yu, K. Value of repeated fine needle aspirations of the thyroid: An analysis of over ten thousand FNAs. Thyroid 2007, 17, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Mihai, R.; Parker, A.J.; Roskell, D.; Sadler, G.P. One in four patients with follicular thyroid cytology (THY3) has a thyroid carcinoma. Thyroid 2009, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Gharib, H. Fine-needle aspiration biopsy of thyroid nodules: Advantages, limitations and effects. Mayo Clin. Proc. 1994, 69, 44–50. [Google Scholar] [CrossRef]
- Cibas, E.S.; Ali, S.Z. The Bethesda System for Reporting Thyroid Cytopathology. Thyroid 2009, 19, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Perros, P.; Boalert, K.; Colley, S.; Evans, C.; Evans, R.M.; Gerrard Ba, G.; Gilbert, J.; Harrison, B.; Johnson, S.J.; Giles, T.E.; et al. British Thyroid Association. Guidelines for the management of thyroid cancer. Clin. Endocrinol. 2014, 61, 1–122. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Nam-Goong, I.S.; Gong, G.; Hong, S.J.; Kim, W.B.; Shong, Y.K. Postoperative findings and risk for malignancy in thyroid nodules with cytological diagnosis of the so-called “follicular neoplasms”. Korean J. Intern. Med. 2003, 18, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Sclabas, G.M.; Staekel, G.A.; Shapiro, S.E.; Fornage, B.D.; Sherman, S.L.; Vassillopoulou-Sellin, R.; Lee, J.E.; Evans, D.B. Fine-needle aspiration of the thyroid and correlation with histopathology in a contemporary serie of 240 patients. Am. J. Surg. 2003, 186, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, S.; Trimboli, P.; Catania, A.; Ulisse, S.; De Antoni, E.; D’Armiento, M. Comparison of malignancy rate in thyroid nodules with cytology of indeterminate follicular or indeterminate Hurthle cell neoplasm. Thyroid 2009, 19, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Asari, R.; Niederle, B.E.; Scheuba, C.; Riss, P.; Koperek, O.; Kaserer, K.; Niederle, B. Indeterminate thyroid nodules: A challenge for the surgical strategy. Surgery 2010, 148, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Musholt, T.J.; Clerici, T.; Dralle, H.; Frilling, A.; Goretzki, P.E.; Hermann, M.M.; Kußmann, J.; Lorenz, K.; Nies, C.; Schabram, J.; et al. German Association of Endocrine Surgeons practice guidelines for the surgical treatment of benign thyroid disease. Langenbecks Arch. Surg. 2011, 396, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Bartolazzi, A.; Gasbarri, A.; Papotti, M.; Bussolati, G.; Lucante, T.; Khan, A.; Inohara, H.; Marandino, F.; Orlandi, F.; Nardi, F.; et al. Application of immunodiagnostic method for improving preoperative diagnosis of nodular thyroid lesions. Lancet 2001, 357, 1644–1650. [Google Scholar] [CrossRef]
- Bartolazzi, A.; Orlandi, F.; Saggiorato, E.; Volante, M.; Arecco, F.; Rossetto, R.; Palestini, N.; Ghigo, E.; Papotti, M.; Bussolati, G.; et al. Galectin-3-expression analysis in the surgical selection of follicular thyroid nodules with indeterminate fine-needle aspiration cytology: A prospective multicenter study. Lancet Oncol. 2008, 9, 543–549. [Google Scholar] [CrossRef]
- Xu, X.C.; el-Naggar, A.K.; Lotan, R. Differential expression of Galectin-1 and Galectin-3 in thyroid tumors. Potential diagnostic implications. Am. J. Pathol. 1995, 147, 815–822. [Google Scholar] [PubMed]
- Gasbarri, A.; Martegani, M.P.; Del Prete, F.; Lucante, T.; Natali, P.G.; Bartolazzi, A. Galectin-3 and CD44v6 isoforms in the pre-operative evaluation of thyroid nodules. J. Clin. Oncol. 1999, 17, 3494–3502. [Google Scholar] [CrossRef] [PubMed]
- Saggiorato, E.; Cappia, S.; De Giuli, P.; Mussa, A.; Pancani, G.; Caraci, P.; Angeli, A.; Orlandi, F. Galectin-3 as a presurgical immunocytodiagnostic marker of minimally invasive follicular thyroid carcinoma. J. Clin. Endocrinol. Metab. 2001, 86, 5152–5158. [Google Scholar] [CrossRef] [PubMed]
- Papotti, M.; Rodriguez, J.; De Pompa, R.; Bartolazzi, A.; Rosai, J. Galectin-3 and HBME-1 expression in well-differentiated thyroid tumors with follicular architecture of uncertain malignant potential. Mod. Pathol. 2005, 18, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Carpi, A.; Naccarato, A.G.; Iervasi, G.; Nicolini, A.; Bevilacqua, G.; Viacava, P.; Collecchi, P.; Lavra, L.; Marchetti, C.; Sciacchitano, S.; et al. Large needle aspiration biopsy and galectin-3 determination in selected thyroid nodules with indeterminate FNA cytology. Br. J. Cancer 2006, 95, 204–209. [Google Scholar] [CrossRef] [PubMed]
- De Matos, L.L.; Del Giglio, A.B.; Matsubayashi, C.O.; de Lima Farah, M.; Del Giglio, A.; da Silva Pinhal, M.A. Expression of CK-19, galectin-3 and HBME-1 in the differentiation of thyroid lesions: Systematic review and diagnostic meta-analysis. Diagn. Pathol. 2012, 7, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, M.E.; LiVolsi, V.A.; Pasha, T.L.; Roberts, S.A.; Wojcik, E.M.; Baloch, Z.W. Immunohistochemical Expression of Galectin-3 in Benign and Malignant Thyroid Lesions. Arch. Pathnol. Lab. Med. 2002, 126, 710–713. [Google Scholar]
- Carpi, A.; Rossi, G.; Di Coscio, G.D.; Iervasi, G.; Nicolini, A.; Carpi, F.; Mechanick, J.I.; Bartolazzi, A. Galectin-3 detection on large-needle aspiration biopsy improves preoperative selection of thyroid nodules: A prospective cohort study. Ann. Med. 2010, 42, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Saggiorato, E.; De Pompa, R.; Volante, M.; Cappia, S.; Arecco, F.; Dei Tos, A.P.; Orlandi, F.; Papotti, M. Characterization of thyroid ‘follicular neoplasms’ in fine-needle aspiration cytological specimens using a panel of immunohistochemical markers: A proposal for clinical application. Endocr.-Relat. Cancer 2005, 12, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.B.; Shroyer, K.R.; Heinz, D.E.; Nawaz, S.; Said, M.S.; Haugen, B.R. The use of combination of galectin-3 and thyroid peroxidase for the diagnosis and prognosis of thyroid cancer. Am. J. Clin. Pathol. 2004, 122, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.L.; Chiu, C.G.; Gown, A.M.; Jones, S.J.; Wiseman, S.M. Biomarker panel diagnosis of thyroid cancer: A critical review. Expert Rev. Anticancer Ther. 2008, 8, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.G.; Strugnell, S.S.; Griffith, O.L.; Jones, S.J.; Gown, A.M.; Walker, B.; Nabi, I.R.; Wiseman, S.M. Diagnostic utility of galectin-3 in thyroid cancer. Am. J. Pathol. 2010, 176, 2067–2081. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, Y.; Inohara, H.; Yoshii, T.; Oshima, K.; Nakahara, S.; Akahani, S.; Honjo, Y.; Yamamoto, Y.; Raz, A.; Kubo, T. Malignant transformation of thyroid follicular cells by galectin-3. Cancer Lett. 2003, 195, 111–119. [Google Scholar] [CrossRef]
- Yoshii, T.; Inohara, H.; Takenaka, Y.; Honjo, Y.; Akahani, S.; Nomura, T.; Raz, A.; Kubo, T. Galectin-3 maintains the transformed phenotype of thyroid papillary carcinoma cells. Int. J. Oncol. 2001, 18, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Honjo, Y.; Nangia-Makker, P.; Inohara, H.; Raz, A. Down-regulation of galectin-3 suppresses tumorigenicity of human breast carcinoma cells. Clin. Cancer Res. 2001, 7, 661–668. [Google Scholar] [PubMed]
- Akahani, S.; Nangia-Makker, P.; Inohara, H.; Kim, H.R.; Raz, A. Galectin-3: A novel antiapoptotic molecule with a functional BH1 (NWGR) domain of Bcl-2 family. Cancer Res. 1997, 57, 5272–5276. [Google Scholar] [PubMed]
- Cecchinelli, B.; Lavra, L.; Rinaldo, C.; Iacovelli, S.; Gurtner, A.; Gasbarri, A.; Ulivieri, A.; Del Prete, F.; Trovato, M.; Piaggio, G.; et al. Repression of the antiapoptotic molecule galectin-3 by homeodomain-interacting protein kinase 2-activated p53 is required for p53-induced apoptosis. Mol. Cell. Biol. 2006, 26, 4746–4757. [Google Scholar] [CrossRef] [PubMed]
- Lavra, L.; Rinaldo, C.; Ulivieri, A.; Luciani, E.; Fidanza, P.; Giacomelli, L.; Bellotti, C.; Ricci, A.; Trovato, M.; Soddu, S.; et al. The loss of the p53 activator HIPK2 is responsible for galectin-3 overexpression in well differentiated thyroid carcinomas. PLoS ONE 2011, 6, e20665. [Google Scholar] [CrossRef] [PubMed]
- Keller, S.; Angrisano, T.; Florio, E.; Pero, R.; Decaussin-Petrucci, M.; Troncone, G.; Capasso, M.; Lembo, F.; Fusco, A.; Chiarotti, L. DNA methylation state of the galectin-3 gene represents a potential new marker of thyroid malignancy. Oncol. Lett. 2013, 6, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Rabinovich, G.A. Galectins as modulators of tumor progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Lavra, L.; Ulivieri, A.; Magi, F.; Porcelli, T.; Amendola, S.; De Francesco, G.P.; Bellotti, C.; Trovato, M.C.; Salehi, L.B.; et al. Combined clinical and ultrasound follow-up assists in malignancy detection in Galectin-3 negative Thy-3 thyroid nodules. Endocrine 2016, 54, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Bartolazzi, A.; Bellotti, C.; Sciacchitano, S. Methodology and technical requirements of the galectin-3 test for the preoperative characterization of thyroid nodules. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 2–7. [Google Scholar] [CrossRef] [PubMed]
- The American Thyroid Association. Consensus Guidelines for Thyroid Testing in the New Millennium: In Laboratory Medicine Practice Guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. (Section H): Thyroid fine needle aspiration (FNA) and cytology. Thyroid 2003, 13, 80–86. [Google Scholar]
- Cooper, D.S.; Doherty, G.M.; Haugen, B.R.; Kloos, R.T.; Lee, S.L.; Mandel, S.J.; Mazzaferri, E.L.; McIver, B.; Pacini, F.; Schlumberger, M.; et al. Revised American Thyroid Association Management Guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009, 19, 1167–1214. [Google Scholar] [CrossRef] [PubMed]
- Trimboli, P.; Virili, C.; Romanelli, F.; Crescenzi, A.; Giovanella, L. Galectin-3 Performance in Histologic a Cytologic Assessment of Thyroid Nodules: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2017, 18, 1756. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.K.; Kennedy, G.C.; Baloch, Z.W.; Cibas, E.S.; Chudova, D.; Diggans, J.; Friedman, L.; Kloos, R.T.; LiVolsi, V.A.; Mandel, S.J.; et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N. Engl. J. Med. 2012, 367, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Nikiforov, Y.E.; Carty, S.E.; Chiosea, S.I.; Coyne, C.; Duvvuri, U.; Ferris, R.L.; Gooding, W.E.; LeBeau, S.O.; Ohri, N.P.; Seethala, R.R.; et al. Impact of the Multi-Gene Thyroseq Next-Generation Sequencing Assay on Cancer Diagnosis in Thyroid nodules with atypia of undeterminate significance/Follicular lesion of undeterminate significance cytology. Thyroid 2015, 25, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Sciacchitano, S.; Lavra, L.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Trovato, M.; Drago, C.; Bartolazzi, A. Comparative analysis of diagnostic performance, feasibility and cost of different test-methods for thyroid nodules with indeterminate cytology. Oncotarget 2017, 8, 49421–49442. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Castaldi, P.; Villani, M.F.; Perotti, G.; de Waure, C.; Filice, A.; Ambrosini, V.; Cremonini, N.; Santimaria, M.; Versari, A.; et al. Comparison of 18F-DOPA, 18F-FDG and 68Ga-somatostatin analogue PET/CT in patients with recurrent medullary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Traugott, A.L.; Dehdashti, F.; Trinkaus, K.; Cohen, M.; Fialkowski, E.; Quayle, F.; Hussain, H.; Davila, R.; Ylagan, L.; Moley, J.F. Exclusion of malignancy in thyroid nodules with indeterminate fine-needle aspiration cytology after negative 18F-fluorodeoxyglucose positron emission tomography: Interim analysis. World J. Surg. 2010, 34, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Deandreis, D.; Al Ghuzlan, A.; Auperin, A.; Vielh, P.; Caillou, B.; Chami, L.; Lumbroso, J.; Travagli, J.P.; Hartl, D.; Baudin, E.; et al. Is (18)F-fluorodeoxyglucose-PET/CT useful for the presurgical characterization of thyroid nodules with indeterminate fine needle aspiration cytology? Thyroid 2012, 22, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Vriens, D.; de Wilt, J.H.; van der Wilt, G.I.; Netea-Maier, R.T.; Oyen, W.J.; de Geus-Oei, L.F. The role of [18F]-2-fluoro-2-deoxy-d-glucose-positron emission tomography in thyroid nodules with indeterminate fine-needle aspiration biopsy: Systematic review and meta-analysis of the literature. Cancer 2011, 117, 4582–4594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartolazzi, A.; D’Alessandria, C.; Parisella, M.G.; Signore, A.; Del Prete, F.; Lavra, L.; Braesch-Andersen, S.; Massari, R.; Trotta, C.; Soluri, A.; et al. Thyroid cancer imaging in vivo by targeting the anti-apoptotic molecule galectin-3. PLoS ONE 2008, 3, e3768. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandria, C.; Braesch-Andersen, S.; Bejo, K.; Reder, S.; Blechert, B.; Schwaiger, M.; Bartolazzi, A. Noninvasive in Vivo Imaging and Biologic Characterization of Thyroid Tumors by ImmunoPET Targeting of Galectin-3. Cancer Res. 2016, 76, 3583–3592. [Google Scholar] [CrossRef] [PubMed]
- Hodak, S.P.; Rosenthal, D.S.; American Thyroid Association Clinical Affairs Committee. Information for clinicians: Commercially available molecular diagnosis testing in the evaluation of thyroid nodule fine-needle aspiration specimens. Thyroid 2013, 23, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Facey, K.; Bradbury, I.; Laking, G.; Payne, E. Overview of the clinical effectiveness of positron emission tomography imaging in selected cancers. Health Technol. Assess. 2007, 11. [Google Scholar] [CrossRef]
- Buck, A.K.; Herrmann, K.; Stargardt, T.; Dechow, T.; Krause, B.J.; Schreyogg, J. Economic evaluation of PET and PET/CT in oncology: Evidence and methodologic approaches. J. Nucl. Med. Technol. 2010, 38, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Kindt, N.; Journe, F.; Ghanem, G.E.; Saussez, S. Galectins and carcinogenesis: Their role in head and neck carcinomas and thyroid carcinomas. Int. J. Mol. Sci. 2017, 18, 2745. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Francuz, T.; Gallego-Colon, E.; Ruiz-Agamez, N.; Kubeczko, M.; Grochola, I.; Wojnar, J. Galectin targeted therapy in oncology: Current knowledge and perspectives. Int. J. Mol. Sci. 2018, 19, 210. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.; Biran, A.; Poirier, F.; Raz, A.; Kloog, Y. Galectin-3 mediates cross-talk between k-Ras and Let-7c tumor suppressor microRNA. PLoS ONE 2011, 6, e27490. [Google Scholar] [CrossRef] [PubMed]



| A: A purified and well-characterized mAb to human galectin-3 (concentration ranging from 5–10 μg/mL) must be used in immunohisto-cytochemistry (direct or indirect immunoperoxidase) with a biotin-free detection system. |
| B: Galectin-3 immunostaining must be applied on formalin-fixed and paraffin embedded cyto-histological substrates (i.e., FNA-derived cellblocks). |
| C: Antigen retrieval microwave treatment with 0.01 M citrate buffer, pH6 for three cycles of 3–5 min each at 750 W is necessary. |
| D: Follicular thyroid cells showing galectin-3 accumulation in the cytoplasm, with or without nuclear staining, are considered positive. Scattered foamy macrophages serve as the internal positive control. |
| E: In Hashimoto’s thyroiditis (HT) and less frequently in chronic lymphocytic thyroiditis, false positive immunostaining for galectin-3 may occur in follicular thyroid cells within inflammatory follicles. This may generate false positive results (nodular lesions in HT always require a multidisciplinary clinical-pathological evaluation for a better therapeutic decision). |
| F: The surgical option for galectin-3-positive cases is advisable, also in the presence of a few galectin-3-positive thyroid follicular cells (cytoplasm +). |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartolazzi, A.; Sciacchitano, S.; D’Alessandria, C. Galectin-3: The Impact on the Clinical Management of Patients with Thyroid Nodules and Future Perspectives. Int. J. Mol. Sci. 2018, 19, 445. https://doi.org/10.3390/ijms19020445
Bartolazzi A, Sciacchitano S, D’Alessandria C. Galectin-3: The Impact on the Clinical Management of Patients with Thyroid Nodules and Future Perspectives. International Journal of Molecular Sciences. 2018; 19(2):445. https://doi.org/10.3390/ijms19020445
Chicago/Turabian StyleBartolazzi, Armando, Salvatore Sciacchitano, and Calogero D’Alessandria. 2018. "Galectin-3: The Impact on the Clinical Management of Patients with Thyroid Nodules and Future Perspectives" International Journal of Molecular Sciences 19, no. 2: 445. https://doi.org/10.3390/ijms19020445
APA StyleBartolazzi, A., Sciacchitano, S., & D’Alessandria, C. (2018). Galectin-3: The Impact on the Clinical Management of Patients with Thyroid Nodules and Future Perspectives. International Journal of Molecular Sciences, 19(2), 445. https://doi.org/10.3390/ijms19020445