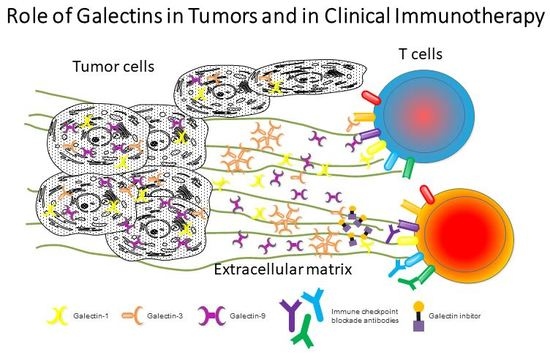
Role of Galectins in Tumors and in Clinical Immunotherapy
Abstract
:1. Introduction
2. Role of Galectins in Tumor Progression and Immune Surveillance
2.1. Human Galectins
2.2. Correlation of Galectin-3 and/or Galectin-1 in Cancers
2.3. Galectin-9 and Tumor Metastasis
3. Galectins Are Involved in Immune Escape by Tumors
3.1. Galectin-1, -3 and -9 Regulate Immune Responses via Different Receptors
3.2. Galectin-1 Modulates the Antitumor T Cell Response
3.3. Double-Edged Sword Role of Galectin-9 in Tumors
4. Targeting Galectins or their Ligands in Preclinical and Clinical Trials
4.1. Galectins Interfere with Chemotherapy against Tumors
4.2. Immunotherapy Combined with Galectin Inhibition
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klibi, J.; Niki, T.; Riedel, A.; Pioche-Durieu, C.; Souquere, S.; Rubinstein, E.; Moulec, S.L.; Guigay, J.; Hirashima, M.; Guemira, F.; et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood 2009, 113, 1957–1966. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, V.L.; Heusschen, R.; Caers, J.; Griffioen, A.W. Galectin expression in cancer diagnosis and prognosis: A systematic review. Biochim. Biophys. Acta 2015, 1855, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, S.; Raz, A. Biological modulation by lectins and their ligands in tumor progression and metastasis. Anticancer Agents Med. Chem. 2008, 8, 22–36. [Google Scholar] [PubMed]
- Lau, K.S.; Partridge, E.A.; Grigorian, A.; Silvescu, C.I.; Reinhold, V.N.; Demetriou, M.; Dennis, J.W. Complex N-glycan number and degree of branching cooperate to regulate cell proliferation and differentiation. Cell 2007, 129, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Huergo, S.P.; Blidner, A.G.; Rabinovich, G.A. Galectins: Emerging regulatory checkpoints linking tumor immunity and angiogenesis. Curr. Opin. Immunol. 2017, 45, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Toscano, M.A. Turning ‘sweet’ on immunity: Galectin-glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009, 9, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.C.; el-Naggar, A.K.; Lotan, R. Differential expression of galectin-1 and galectin-3 in thyroid tumors. Potential diagnostic implications. Am. J. Pathol. 1995, 147, 815–822. [Google Scholar] [PubMed]
- Van den Brule, F.A.; Buicu, C.; Berchuck, A.; Bast, R.C.; Deprez, M.; Liu, F.T.; Cooper, D.N.; Pieters, C.; Sobel, M.E.; Castronovo, V. Expression of the 67-kD laminin receptor, galectin-1, and galectin-3 in advanced human uterine adenocarcinoma. Hum. Pathol. 1996, 27, 1185–1191. [Google Scholar] [CrossRef]
- Sanjuan, X.; Fernandez, P.L.; Castells, A.; Castronovo, V.; van den Brule, F.; Liu, F.T.; Cardesa, A.; Campo, E. Differential expression of galectin 3 and galectin 1 in colorectal cancer progression. Gastroenterology 1997, 113, 1906–1915. [Google Scholar] [CrossRef]
- Cindolo, L.; Benvenuto, G.; Salvatore, P.; Pero, R.; Salvatore, G.; Mirone, V.; Prezioso, D.; Altieri, V.; Bruni, C.B.; Chiariotti, L. Galectin-1 and galectin-3 expression in human bladder transitional-cell carcinomas. Int. J. Cancer 1999, 84, 39–43. [Google Scholar] [CrossRef]
- Nakamura, M.; Inufusa, H.; Adachi, T.; Aga, M.; Kurimoto, M.; Nakatani, Y.; Wakano, T.; Nakajima, A.; Hida, J.I.; Miyake, M.; et al. Involvement of galectin-3 expression in colorectal cancer progression and metastasis. Int. J. Oncol. 1999, 15, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Guo, X.; Nash, G.B.; Stone, P.C.; Hilkens, J.; Rhodes, J.M.; Yu, L.G. Circulating galectin-3 promotes metastasis by modifying MUC1 localization on cancer cell surface. Cancer Res. 2009, 69, 6799–6806. [Google Scholar] [CrossRef] [PubMed]
- Piyush, T.; Chacko, A.R.; Sindrewicz, P.; Hilkens, J.; Rhodes, J.M.; Yu, L.G. Interaction of galectin-3 with MUC1 on cell surface promotes EGFR dimerization and activation in human epithelial cancer cells. Cell Death Differ. 2017, 24, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.C.; Chen, C.L.; Shan, Y.S.; Lin, C.F. An increase in galectin-3 causes cellular unresponsiveness to IFN-gamma-induced signal transduction and growth inhibition in gastric cancer cells. Oncotarget 2016, 7, 15150–15160. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Alonso, M.; Hirsch, T.; Wildmann, C.; van der Bruggen, P. Galectin-3 captures interferon-gamma in the tumor matrix reducing chemokine gradient production and T-cell tumor infiltration. Nat. Commun. 2017, 8, 793. [Google Scholar] [CrossRef] [PubMed]
- Kageshita, T.; Kashio, Y.; Yamauchi, A.; Seki, M.; Abedin, M.J.; Nishi, N.; Shoji, H.; Nakamura, T.; Ono, T.; Hirashima, M. Possible role of galectin-9 in cell aggregation and apoptosis of human melanoma cell lines and its clinical significance. Int. J. Cancer 2002, 99, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Kasamatsu, A.; Uzawa, K.; Nakashima, D.; Koike, H.; Shiiba, M.; Bukawa, H.; Yokoe, H.; Tanzawa, H. Galectin-9 as a regulator of cellular adhesion in human oral squamous cell carcinoma cell lines. Int. J. Mol. Med. 2005, 16, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Irie, A.; Yamauchi, A.; Kontani, K.; Kihara, M.; Liu, D.; Shirato, Y.; Seki, M.; Nishi, N.; Nakamura, T.; Yokomise, H.; et al. Galectin-9 as a prognostic factor with antimetastatic potential in breast cancer. Clin. Cancer Res. 2005, 11, 2962–2968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Dong, J.H.; Chen, Y.W.; Wang, X.Q.; Li, C.H.; Wang, J.; Wang, G.Q.; Li, H.L.; Wang, X.D. Galectin-9 Acts as a Prognostic Factor with Antimetastatic Potential in Hepatocellular Carcinoma. Asian Pac. J. Cancer Prev. 2012, 13, 2503–2509. [Google Scholar] [CrossRef] [PubMed]
- Nobumoto, A.; Nagahara, K.; Oomizu, S.; Katoh, S.; Nishi, N.; Takeshita, K.; Niki, T.; Tominaga, A.; Yamauchi, A.; Hirashima, M. Galectin-9 suppresses tumor metastasis by blocking adhesion to endothelium and extracellular matrices. Glycobiology 2008, 18, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Walzel, H.; Fahmi, A.A.; Eldesouky, M.A.; Abou-Eladab, E.F.; Waitz, G.; Brock, J.; Tiedge, M. Effects of N-glycan processing inhibitors on signaling events and induction of apoptosis in galectin-1-stimulated Jurkat T lymphocytes. Glycobiology 2006, 16, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Pace, K.E.; Lee, C.; Stewart, P.L.; Baum, L.G. Restricted receptor segregation into membrane microdomains occurs on human T cells during apoptosis induced by galectin-1. J. Immunol. 1999, 163, 3801–3811. [Google Scholar] [PubMed]
- Chung, C.D.; Patel, V.P.; Moran, M.; Lewis, L.A.; Miceli, M.C. Galectin-1 induces partial TCR zeta-chain phosphorylation and antagonizes processive TCR signal transduction. J. Immunol. 2000, 165, 3722–3729. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.H.; Ying, N.W.; Wu, M.H.; Chiang, W.F.; Hsu, C.L.; Wong, T.Y.; Jin, Y.T.; Hong, T.M.; Chen, Y.L. Galectin-1, a novel ligand of neuropilin-1, activates VEGFR-2 signaling and modulates the migration of vascular endothelial cells. Oncogene 2008, 27, 3746–3753. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Rossi, B.; Roux, F.; Termine, E.; Schiff, C. Galectin-1 is a stromal cell ligand of the pre-B cell receptor (BCR) implicated in synapse formation between pre-B and stromal cells and in pre-BCR triggering. Proc. Natl. Acad. Sci. USA 2002, 99, 13014–13019. [Google Scholar] [CrossRef] [PubMed]
- Rossi, B.; Espeli, M.; Schiff, C.; Gauthier, L. Clustering of pre-B cell integrins induces galectin-1-dependent pre-B cell receptor relocalization and activation. J. Immunol. 2006, 177, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Paz, A.; Haklai, R.; Elad-Sfadia, G.; Ballan, E.; Kloog, Y. Galectin-1 binds oncogenic H-Ras to mediate Ras membrane anchorage and cell transformation. Oncogene 2001, 20, 7486–7493. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ruderisch, H.; Detjen, K.M.; Welzel, M.; Andre, S.; Fischer, C.; Gabius, H.J.; Rosewicz, S. Galectin-1 sensitizes carcinoma cells to anoikis via the fibronectin receptor alpha5beta1-integrin. Cell Death Differ. 2011, 18, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Fukumori, T.; Takenaka, Y.; Yoshii, T.; Kim, H.R.; Hogan, V.; Inohara, H.; Kagawa, S.; Raz, A. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003, 63, 8302–8311. [Google Scholar] [PubMed]
- Stillman, B.N.; Hsu, D.K.; Pang, M.; Brewer, C.F.; Johnson, P.; Liu, F.T.; Baum, L.G. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J. Immunol. 2006, 176, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Demetriou, M.; Granovsky, M.; Quaggin, S.; Dennis, J.W. Negative regulation of T-cell activation and autoimmunity by Mgat5 N-glycosylation. Nature 2001, 409, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Fermin, A.; Vardhana, S.; Weng, I.C.; Lo, K.F.; Chang, E.Y.; Maverakis, E.; Yang, R.Y.; Hsu, D.K.; Dustin, M.L.; et al. Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc. Natl. Acad. Sci. USA 2009, 106, 14496–14501. [Google Scholar] [CrossRef] [PubMed]
- Elad-Sfadia, G.; Haklai, R.; Balan, E.; Kloog, Y. Galectin-3 augments K-Ras activation and triggers a Ras signal that attenuates ERK but not phosphoinositide 3-kinase activity. J. Biol. Chem. 2004, 279, 34922–34930. [Google Scholar] [CrossRef] [PubMed]
- Paron, I.; Scaloni, A.; Pines, A.; Bachi, A.; Liu, F.T.; Puppin, C.; Pandolfi, M.; Ledda, L.; Di Loreto, C.; Damante, G.; et al. Nuclear localization of Galectin-3 in transformed thyroid cells: A role in transcriptional regulation. Biochem. Biophys. Res. Commun. 2003, 302, 545–553. [Google Scholar] [CrossRef]
- Inohara, H.; Akahani, S.; Koths, K.; Raz, A. Interactions between galectin-3 and Mac-2-binding protein mediate cell-cell adhesion. Cancer Res. 1996, 56, 4530–4534. [Google Scholar] [PubMed]
- Daley, D.; Mani, V.R.; Mohan, N.; Akkad, N.; Ochi, A.; Heindel, D.W.; Lee, K.B.; Zambirinis, C.P.; Pandian, G.; Savadkar, S.; et al. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat. Med. 2017, 23, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Anderson, D.E.; Bregoli, L.; Hastings, W.D.; Kassam, N.; Lei, C.; Chandwaskar, R.; Karman, J.; Su, E.W.; Hirashima, M.; et al. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science 2007, 318, 1141–1143. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.Y.; Nakagawa, R.; Itoh, A.; Murakami, H.; Kashio, Y.; Abe, H.; Katoh, S.; Kontani, K.; Kihara, M.; Zhang, S.L.; et al. Galectin-9 induces maturation of human monocyte-derived dendritic cells. J. Immunol. 2005, 175, 2974–2981. [Google Scholar] [CrossRef] [PubMed]
- Koguchi, K.; Anderson, D.E.; Yang, L.; O’Connor, K.C.; Kuchroo, V.K.; Hafler, D.A. Dysregulated T cell expression of TIM3 in multiple sclerosis. J. Exp. Med. 2006, 203, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Madireddi, S.; Eun, S.Y.; Lee, S.W.; Nemcovicova, I.; Mehta, A.K.; Zajonc, D.M.; Nishi, N.; Niki, T.; Hirashima, M.; Croft, M. Galectin-9 controls the therapeutic activity of 4-1BB-targeting antibodies. J. Exp. Med. 2014, 211, 1433–1448. [Google Scholar] [CrossRef] [PubMed]
- Vaitaitis, G.M.; Wagner, D.H. Galectin-9 Controls CD40 Signaling through a Tim-3 Independent Mechanism and Redirects the Cytokine Profile of Pathogenic T Cells in Autoimmunity. PLoS ONE 2012, 7, e38708. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, N.; Alvarez, M.; Zwirner, N.W.; Toscano, M.A.; Ilarregui, J.M.; Bravo, A.; Mordoh, J.; Fainboim, L.; Podhajcer, O.L.; Rabinovich, G.A. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell 2004, 5, 241–251. [Google Scholar] [CrossRef]
- Juszczynski, P.; Ouyang, J.; Monti, S.; Rodig, S.J.; Takeyama, K.; Abramson, J.; Chen, W.; Kutok, J.L.; Rabinovich, G.A.; Shipp, M.A. The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc. Natl. Acad. Sci. USA 2007, 104, 13134–13139. [Google Scholar] [CrossRef] [PubMed]
- Cedeno-Laurent, F.; Watanabe, R.; Teague, J.E.; Kupper, T.S.; Clark, R.A.; Dimitroff, C.J. Galectin-1 inhibits the viability, proliferation, and Th1 cytokine production of nonmalignant T cells in patients with leukemic cutaneous T-cell lymphoma. Blood 2012, 119, 3534–3538. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.T.; Shi, G.; Cao, H.; Nelson, D.W.; Wang, Y.; Chen, E.Y.; Zhao, S.; Kong, C.; Richardson, D.; O’Byrne, K.J.; et al. Galectin-1: A link between tumor hypoxia and tumor immune privilege. J. Clin. Oncol. 2005, 23, 8932–8941. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Chen, C.H.; Wang, C.P.; Lin, P.H.; Yang, T.L.; Lou, P.J.; Ko, J.Y.; Wu, C.T.; Chang, Y.L. The immunologic advantage of recurrent nasopharyngeal carcinoma from the viewpoint of Galectin-9/Tim-3-related changes in the tumour microenvironment. Sci. Rep. 2017, 7, 10349. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, K.; Tao, K.; Chen, L.; Zheng, Q.; Lu, X.; Liu, J.; Shi, L.; Liu, C.; Wang, G.; et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus-associated hepatocellular carcinoma. Hepatology 2012, 56, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Nebbia, G.; Peppa, D.; Schurich, A.; Khanna, P.; Singh, H.D.; Cheng, Y.; Rosenberg, W.; Dusheiko, G.; Gilson, R.; ChinAleong, J.; et al. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS ONE 2012, 7, e47648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroda, J.; Yamamoto, M.; Nagoshi, H.; Kobayashi, T.; Sasaki, N.; Shimura, Y.; Horiike, S.; Kimura, S.; Yamauchi, A.; Hirashima, M.; et al. Targeting activating transcription factor 3 by Galectin-9 induces apoptosis and overcomes various types of treatment resistance in chronic myelogenous leukemia. Mol. Cancer Res. 2010, 8, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Kuroda, J.; Ashihara, E.; Oomizu, S.; Terui, Y.; Taniyama, A.; Adachi, S.; Takagi, T.; Yamamoto, M.; Sasaki, N.; et al. Galectin-9 exhibits anti-myeloma activity through JNK and p38 MAP kinase pathways. Leukemia 2010, 24, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Wiersma, V.R.; de Bruyn, M.; van Ginkel, R.J.; Sigar, E.; Hirashima, M.; Niki, T.; Nishi, N.; Samplonius, D.F.; Helfrich, W.; Bremer, E. The glycan-binding protein galectin-9 has direct apoptotic activity toward melanoma cells. J. Investig. Dermatol. 2012, 132, 2302–2305. [Google Scholar] [CrossRef] [PubMed]
- Tadokoro, T.; Morishita, A.; Fujihara, S.; Iwama, H.; Niki, T.; Fujita, K.; Akashi, E.; Mimura, S.; Oura, K.; Sakamoto, T.; et al. Galectin-9: An anticancer molecule for gallbladder carcinoma. Int. J. Oncol. 2016, 48, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Iwama, H.; Sakamoto, T.; Okura, R.; Kobayashi, K.; Takano, J.; Katsura, A.; Tatsuta, M.; Maeda, E.; Mimura, S.; et al. Galectin-9 suppresses the growth of hepatocellular carcinoma via apoptosis in vitro and in vivo. Int. J. Oncol. 2015, 46, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Morishita, A.; Iwama, H.; Fujita, K.; Okura, R.; Fujihara, S.; Yamashita, T.; Fujimori, T.; Kato, K.; Kamada, H.; et al. Galectin-9 suppresses cholangiocarcinoma cell proliferation by inducing apoptosis but not cell cycle arrest. Oncol. Rep. 2015, 34, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Takano, J.; Morishita, A.; Fujihara, S.; Iwama, H.; Kokado, F.; Fujikawa, K.; Fujita, K.; Chiyo, T.; Tadokoro, T.; Sakamoto, T.; et al. Galectin-9 suppresses the proliferation of gastric cancer cells in vitro. Oncol. Rep. 2016, 35, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Iwama, H.; Oura, K.; Tadokoro, T.; Samukawa, E.; Sakamoto, T.; Nomura, T.; Tani, J.; Yoneyama, H.; Morishita, A.; et al. Cancer Therapy Due to Apoptosis: Galectin-9. Int. J. Mol. Sci. 2017, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Szakacs, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Song, L.; Chen, X.L.; Zeng, X.F.; Wu, J.Z.; Zhu, C.R.; Huang, T.; Tan, X.P.; Lin, X.M.; Yang, Q.; et al. Identification of galectin-1 as a novel mediator for chemoresistance in chronic myeloid leukemia cells. Oncotarget 2016, 7, 26709–26723. [Google Scholar] [CrossRef] [PubMed]
- Harazono, Y.; Kho, D.H.; Balan, V.; Nakajima, K.; Hogan, V.; Raz, A. Extracellular galectin-3 programs multidrug resistance through Na+/K+-ATPase and P-glycoprotein signaling. Oncotarget 2015, 6, 19592–19604. [Google Scholar] [CrossRef] [PubMed]
- Lykken, J.M.; Horikawa, M.; Minard-Colin, V.; Kamata, M.; Miyagaki, T.; Poe, J.C.; Tedder, T.F. Galectin-1 drives lymphoma CD20 immunotherapy resistance: Validation of a preclinical system to identify resistance mechanisms. Blood 2016, 127, 1886–1895. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef] [PubMed]
- Romero, D. Immunotherapy: PD-1 says goodbye, TIM-3 says hello. Nat. Rev. Clin. Oncol. 2016, 13, 202–203. [Google Scholar] [CrossRef] [PubMed]
| Ligand | Targeted Cells | Biological Function | Refs. | |
|---|---|---|---|---|
| Galectin-1 | CD2, CD3, CD7, CD43 and CD45 | T cells | Apoptosis | [21,22] |
| TCR | T cells | Signal transduction | [23] | |
| Neuropilin 1 | Endothelial cells | Cell migration | [24] | |
| Pre-BCR | Pre-B cells | Signal transduction Cell maturation | [25,26] | |
| α4 integrins | Pre-B cells | Signal transduction Cell maturation | [25,26] | |
| H-Ras | Endometrial cancer cells | Membrane anchorage Cell transformation | [27] | |
| α5β1- integrins | Epithelial cancer cells | Epithelial integrity | [28] | |
| Galectin-3 | CD7, CD29, CD45, CD71 | T cells | Apoptosis | [29,30] |
| TCR | T cells | Signal transduction | [31] | |
| Alix | T cells | Signal transduction | [32] | |
| MUC1 | Epithelial cancer cells | Signal transduction | [13] | |
| K-Ras | Breast carcinoma cells | Enhanced K-Ras stability | [33] | |
| TTF-1 | Thyroid cancer cells | Tumor progression | [34] | |
| Mac-2BP | Melanoma cells | Cell-cell adhesion | [35] | |
| Galectin-9 | Dectin-1 | Macrophages | Tolerogenic macrophage programming and adaptive immune suppression | [36] |
| TIM-3 | Dendritic cells, monocytes | Maturation and cytokine production | [37,38] | |
| TIM-3 | T cells | Apoptosis | [39] | |
| 4-1BB | T cells | Signal transduction | [40] | |
| CD40 | T cells | Inducing cell death and suppressing proliferation | [41] |
| Status | Condition | Intervention | Phase | Sponsors and Collaborators |
|---|---|---|---|---|
| Completed | Colorectal, lung, breast, head and neck, and prostate cancers | GM-CT-01 (galectin-3 inhibitor) combined with 5-fluorouracil | Phase I NCT00054977 | Galectin Therapeutics Inc. |
| Withdrawn | Cancers of the bile duct and gallbladder | GM-CT-01 (galectin-3 inhibitor) combined with 5-fluorouracil | Phase II NCT00386516 | Galectin Therapeutics Inc. |
| Terminated | Colorectal cancer | GM-CT-01 (galectin-3 inhibitor) combined with 5-fluorouracil, leukovorin, bevacizumab | Phase II NCT00388700 | Galectin Therapeutics Inc. |
| Unknown status | Metastatic melanoma | Tumor peptide vaccination combined with GM-CT-01 (galectin-3 inhibitor) | Phase I/II NCT01723813 | Cliniques Universitaires Saint-Luc Université Catholique de Louvain |
| Unknown status | Solid tumors | OTX008 inhibitor of galectin-1 expression | Phase I NCT01724320 | Oncoethix GmbH |
| Withdrawn | Diffuse large B-cell lymphoma | GCS-100 (galectin-3 inhibitor) | Phase I/II NCT00776802 | La Jolla Pharmaceutical Co. investigators |
| Recruiting | Metastatic melanoma | GR-MD-02 (galectin-3 inhibitor) combined with ipilimumab (anti-CTLA-4) | Phase IB NCT02117362 | Providence Health & Services, Providence Cancer Center, Earle A. Chiles Research Institute; Galectin Therapeutics Inc. |
| Recruiting | Melanoma, non-small cell lung cancer, and squamous cell head and neck cancers | GR-MD-02 combined with pembrolizumab (anti-PD-1; keytruda) | Phase IB NCT02575404 | Galectin Therapeutics Inc.; Providence Health & Services |
| Recruiting | Advanced or metastatic solid tumors | TSR-022 (anti-TIM-3) combined with anti-PD-1 antibody | Phase I NCT02817633 | Tesaro, Inc. |
| Recruiting | Solid tumors | LY3321367 (anti-TIM-3) combined with LY3300054 (anti-PD-L1) | Phase I NCT03099109 | Eli Lilly and Co. |
| Recruiting | Advanced Malignancies | MBG453 (anti-TIM-3 ) combined with PDR001 (anti-PD-1) | Phase I/II NCT02608268 | Novartis Pharmaceuticals |
| Recruiting | relapsed/refractory acute myeloid leukemia | MBG453 (anti-TIM-3 ) combined with PDR001 (anti-PD-1) and/or decitabine (5-aza-2′-deoxycytidine) | Phase I NCT03066648 | Novartis Pharmaceuticals |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, F.-C.; Chen, H.-Y.; Kuo, C.-C.; Sytwu, H.-K. Role of Galectins in Tumors and in Clinical Immunotherapy. Int. J. Mol. Sci. 2018, 19, 430. https://doi.org/10.3390/ijms19020430
Chou F-C, Chen H-Y, Kuo C-C, Sytwu H-K. Role of Galectins in Tumors and in Clinical Immunotherapy. International Journal of Molecular Sciences. 2018; 19(2):430. https://doi.org/10.3390/ijms19020430
Chicago/Turabian StyleChou, Feng-Cheng, Heng-Yi Chen, Chih-Chi Kuo, and Huey-Kang Sytwu. 2018. "Role of Galectins in Tumors and in Clinical Immunotherapy" International Journal of Molecular Sciences 19, no. 2: 430. https://doi.org/10.3390/ijms19020430
APA StyleChou, F. -C., Chen, H. -Y., Kuo, C. -C., & Sytwu, H. -K. (2018). Role of Galectins in Tumors and in Clinical Immunotherapy. International Journal of Molecular Sciences, 19(2), 430. https://doi.org/10.3390/ijms19020430