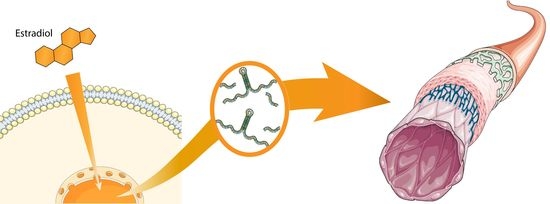
miRNA as a New Regulatory Mechanism of Estrogen Vascular Action
Abstract
:1. Introduction
2. Role of Estrogen in Vascular Physiology
3. miRNA as Epigenetic Regulatory Mechanism
4. Vascular miRNA and Estrogen Action
4.1. Estrogen-Dependent miRNA and Cardiovascular Function
4.2. miRNA and Hormone Replacement Therapy
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burns, K.A.; Korach, K.S. Estrogen receptors and human disease: An update. Arch. Toxicol. 2012, 86, 1491–1504. [Google Scholar] [CrossRef] [PubMed]
- Vrtacnik, P.; Ostanek, B.; Mencej-Bedrac, S.; Marc, J. The many faces of estrogen signaling. Biochem. Med. 2014, 24, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Deroo, B.J.; Korach, K.S. Estrogen receptors and human disease. J. Clin. Investig. 2006, 116, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, M.E.; Karas, R.H. Molecular and Cellular Basis of Cardiovascular Gender Differences. Science 2005, 308, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Hayward, C.S.; Kelly, R.P.; Collins, P. The roles of gender, the menopause and hormone replacement on cardiovascular function. Cardiovasc. Res. 2000, 46, 28–49. [Google Scholar] [CrossRef]
- Mendelsohn, M.E.; Karas, R.H. The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 1999, 340, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, T.S.; Gissler, M.; Merikukka, M.; Tuomikoski, P.; Ylikorkala, O. Sex differences in age-related cardiovascular mortality. PLoS ONE 2013, 8, e63347. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Hsia, J.; Cauley, J.A.; Richards, C.; Harris, F.; Fong, J.; Barrett-Connor, E.; Hulley, S.B. Postmenopausal hormone therapy and risk of stroke: The Heart and Estrogen-progestin Replacement Study (HERS). Circulation 2001, 103, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Rossouw, J.E.; Prentice, R.L.; Manson, J.E.; Wu, L.; Barad, D.; Barnabei, V.M.; Ko, M.; LaCroix, A.Z.; Margolis, K.L.; Stefanick, M.L. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007, 297, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Lobo, R.A. Hormone-replacement therapy: Current thinking. Nat. Rev. Endocrinol. 2017, 13, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.B.; Melendez, G.C.; Appt, S.E. Timing hypothesis for postmenopausal hormone therapy: Its origin, current status, and future. Menopause 2013, 20, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, A.; Bower, J.K.; McFetridge-Durdle, J.; Blumenthal, J.A.; Newby, L.K.; Hinderliter, A.L. Age moderates the short-term effects of transdermal 17β-estradiol on endothelium-dependent vascular function in postmenopausal women. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1782–1787. [Google Scholar] [CrossRef] [PubMed]
- Novella, S.; Heras, M.; Hermenegildo, C.; Dantas, A.P. Effects of estrogen on vascular inflammation: A matter of timing. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2035–2042. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.A. Estrogen, vascular estrogen receptor and hormone therapy in postmenopausal vascular disease. Biochem. Pharmacol. 2013, 86, 1627–1642. [Google Scholar] [CrossRef] [PubMed]
- Härkönen, P.L.; Väänänen, H.K. Monocyte—Macrophage System as a Target for Estrogen and Selective Estrogen Receptor Modulators. Ann. N. Y. Acad. Sci. 2006, 1089, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001, 29, 2905–2919. [Google Scholar] [CrossRef] [PubMed]
- O’Lone, R.; Knorr, K.; Jaffe, I.Z.; Schaffer, M.E.; Martini, P.G.; Karas, R.H.; Bienkowska, J.; Mendelsohn, M.E.; Hansen, U. Estrogen receptors α and β mediate distinct pathways of vascular gene expression, including genes involved in mitochondrial electron transport and generation of reactive oxygen species. Mol. Endocrinol. 2007, 21, 1281–1296. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, M.K.; Moverare, S.; Skrtic, S.; Gao, H.; Dahlman-Wright, K.; Gustafsson, J.A.; Ohlsson, C. Estrogen receptor (ER)-β reduces ERα-regulated gene transcription, supporting a “ying yang” relationship between ERα and ERβ in mice. Mol. Endocrinol. 2003, 17, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, S.; Zhang, X.; Takata, K.; Takahashi, K.; Karas, R.H.; Kurachi, H.; Mendelsohn, M.E. Differential regulation of the inducible nitric oxide synthase gene by estrogen receptors 1 and 2. J. Endocrinol. 2008, 199, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Lahm, T.; Crisostomo, P.R.; Markel, T.A.; Wang, M.; Wang, Y.; Tan, J.; Meldrum, D.R. Selective estrogen receptor-α and estrogen receptor-β agonists rapidly decrease pulmonary artery vasoconstriction by a nitric oxide-dependent mechanism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1486–R1493. [Google Scholar] [CrossRef] [PubMed]
- Arias-Loza, P.A.; Hu, K.; Dienesch, C.; Mehlich, A.M.; Konig, S.; Jazbutyte, V.; Neyses, L.; Hegele-Hartung, C.; Heinrich Fritzemeier, K.; Pelzer, T. Both estrogen receptor subtypes, α and β, attenuate cardiovascular remodeling in aldosterone salt-treated rats. Hypertension 2007, 50, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.; Steenbergen, C. Estrogen regulation of protein expression and signaling pathways in the heart. Biol. Sex Differ. 2014, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Pare, G.; Krust, A.; Karas, R.H.; Dupont, S.; Aronovitz, M.; Chambon, P.; Mendelsohn, M.E. Estrogen receptor-α mediates the protective effects of estrogen against vascular injury. Circ. Res. 2002, 90, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Arnal, J.-F.; Lenfant, F.; Metivier, R.; Flouriot, G.; Henrion, D.; Adlanmerini, M.; Fontaine, C.; Gourdy, P.; Chambon, P.; Katzenellenbogen, B.; et al. Membrane and Nuclear Estrogen Receptor α Actions: From Tissue Specificity to Medical Implications. Physiol. Rev. 2017, 97, 1045. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R. Plasma membrane estrogen receptors. Trends Endocrinol. Metab. 2009, 20, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Prossnitz, E.R.; Barton, M. The G protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011, 7, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Vitale, C.; Mendelsohn, M.E.; Rosano, G.M. Gender differences in the cardiovascular effect of sex hormones. Nat. Rev. Cardiol. 2009, 6, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.M.; Duckles, S.P. Vascular actions of estrogens: Functional implications. Pharmacol. Rev. 2008, 60, 210–241. [Google Scholar] [CrossRef] [PubMed]
- Barton, M. Cholesterol and atherosclerosis: Modulation by oestrogen. Curr. Opin. Lipidol. 2013, 24, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Hirose, M.; Kageyama, K. Roles of Oxidative Stress and Redox Regulation in Atherosclerosis. J. Atheroscler. Thromb. 2009, 16, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.M.; Mulvagh, S.L. Sex steroids and endothelial function: Translating basic science to clinical practice. Trends Pharmacol. Sci. 2007, 28, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Gisclard, V.; Flavahan, N.A.; Vanhoutte, P.M. α adrenergic responses of blood vessels of rabbits after ovariectomy and administration of 17 β-estradiol. J. Pharmacol. Exp. Ther. 1987, 240, 466–470. [Google Scholar] [PubMed]
- Chambliss, K.L.; Shaul, P.W. Estrogen modulation of endothelial nitric oxide synthase. Endocr. Rev. 2002, 23, 665–686. [Google Scholar] [CrossRef] [PubMed]
- Novella, S.; Laguna-Fernández, A.; Lázaro-Franco, M.; Sobrino, A.; Bueno-Betí, C.; Tarín, J.J.; Monsalve, E.; Sanchís, J.; Hermenegildo, C. Estradiol, acting through estrogen receptor α, restores dimethylarginine dimethylaminohydrolase activity and nitric oxide production in oxLDL-treated human arterial endothelial cells. Mol. Cell. Endocrinol. 2013, 365, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Sobrino, A.; Vallejo, S.; Novella, S.; Lazaro-Franco, M.; Mompeon, A.; Bueno-Beti, C.; Walther, T.; Sanchez-Ferrer, C.; Peiro, C.; Hermenegildo, C. Mas receptor is involved in the estrogen-receptor induced nitric oxide-dependent vasorelaxation. Biochem. Pharmacol. 2017, 129, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Sobrino, A.; Oviedo, P.J.; Novella, S.; Laguna-Fernandez, A.; Bueno, C.; Garcia-Perez, M.A.; Tarin, J.J.; Cano, A.; Hermenegildo, C. Estradiol selectively stimulates endothelial prostacyclin production through estrogen receptor-α. J. Mol. Endocrinol. 2010, 44, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Mompeón, A.; Lázaro-Franco, M.; Bueno-Betí, C.; Pérez-Cremades, D.; Vidal-Gómez, X.; Monsalve, E.; Gironacci, M.M.; Hermenegildo, C.; Novella, S. Estradiol, acting through ERα, induces endothelial non-classic renin-angiotensin system increasing angiotensin 1–7 production. Mol. Cell. Endocrinol. 2016, 422, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.K.; Jackson, E.K.; Keller, P.J.; Imthurn, B.; Rosselli, M. Estradiol metabolites inhibit endothelin synthesis by an estrogen receptor-independent mechanism. Hypertension 2001, 37, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Nickenig, G.; Bäumer, A.T.; Grohè, C.; Kahlert, S.; Strehlow, K.; Rosenkranz, S.; Stäblein, A.; Beckers, F.; Smits, J.F.M.; Daemen, M.J.A.P.; et al. Estrogen modulates AT 1 receptor gene expression in vitro and in vivo. Circulation 1998, 97, 2197. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef] [PubMed]
- Bakir, S.; Mori, T.; Durand, J.; Chen, Y.F.; Thompson, J.A.; Oparil, S. Estrogen-induced vasoprotection is estrogen receptor dependent: Evidence from the balloon-injured rat carotid artery model. Circulation 2000, 101, 2342–2344. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.P.; Feng, W.; Xing, D.; Weathington, N.M.; Blalock, J.E.; Chen, Y.F.; Oparil, S. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation 2004, 110, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Miller, A.; Novak, L.; Rocha, R.; Chen, Y.F.; Oparil, S. Estradiol and progestins differentially modulate leukocyte infiltration after vascular injury. Circulation 2004, 109, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Caulin-Glaser, T.; Watson, C.A.; Pardi, R.; Bender, J.R. Effects of 17β-estradiol on cytokine-induced endothelial cell adhesion molecule expression. J. Clin. Investig. 1996, 98, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, A.; Hermenegildo, C.; Issekutz, A.C.; Esplugues, J.V.; Sanz, M.J. Estrogens inhibit angiotensin II-induced leukocyte-endothelial cell interactions in vivo via rapid endothelial nitric oxide synthase and cyclooxygenase activation. Circ. Res. 2002, 91, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, T.S.; St Clair, R.W. Estradiol reduces basal and cytokine induced monocyte adhesion to endothelial cells. Maturitas 2002, 41, 313–319. [Google Scholar] [CrossRef]
- Abu-Taha, M.; Rius, C.; Hermenegildo, C.; Noguera, I.; Cerda-Nicolas, J.-M.; Issekutz, A.C.; Jose, P.J.; Cortijo, J.; Morcillo, E.J.; Sanz, M.-J. Menopause and Ovariectomy Cause a Low Grade of Systemic Inflammation that May Be Prevented by Chronic Treatment with Low Doses of Estrogen or Losartan. J. Immunol. 2009, 183, 1393. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Durand, J.; Chen, Y.; Thompson, J.A.; Bakir, S.; Oparil, S. Effects of short-term estrogen treatment on the neointimal response to balloon injury of rat carotid artery. Am. J. Cardiol. 2000, 85, 1276–1279. [Google Scholar] [CrossRef]
- Terauchi, M.; Honjo, H.; Mizunuma, H.; Aso, T. Effects of oral estradiol and levonorgestrel on cardiovascular risk markers in postmenopausal women. Arch. Gynecol. Obstet. 2012, 285, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, R.S.; Born, K.M. Effect of estrogen and progesterone on the hepatic cholesterol 7-α-hydroxylase activity in ovariectomized baboons. Biochim. Biophys. Acta 1991, 1084, 300–302. [Google Scholar] [CrossRef]
- Walsh, B.W.; Schiff, I.; Rosner, B.; Greenberg, L.; Ravnikar, V.; Sacks, F.M. Effects of postmenopausal estrogen replacement on the concentrations and metabolism of plasma lipoproteins. N. Engl. J. Med. 1991, 325, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- McCrohon, J.A.; Nakhla, S.; Jessup, W.; Stanley, K.K.; Celermajer, D.S. Estrogen and progesterone reduce lipid accumulation in human monocyte-derived macrophages: A sex-specific effect. Circulation 1999, 100, 2319–2325. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, Y.; Zhu, L.; Wang, W.; Wan, Z.; Chen, F.; Wu, Y.; Zhou, J.; Yuan, Z. 17β-estradiol promotes cholesterol efflux from vascular smooth muscle cells through a liver X receptor α-dependent pathway. Int. J. Mol. Med. 2014, 33, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Gardner, G.; Banka, C.L.; Roberts, K.A.; Mullick, A.E.; Rutledge, J.C. Modified LDL-mediated increases in endothelial layer permeability are attenuated with 17 β-estradiol. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Florian, M.; Magder, S. Estrogen decreases TNF-α and oxidized LDL induced apoptosis in endothelial cells. Steroids 2008, 73, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Walsh, B.A.; Mullick, A.E.; Walzem, R.L.; Rutledge, J.C. 17β-estradiol reduces tumor necrosis factor-α-mediated LDL accumulation in the artery wall. J. Lipid Res. 1999, 40, 387–396. [Google Scholar] [PubMed]
- Mooradian, A.D. Antioxidant properties of steroids. J. Steroid Biochem. Mol. Biol. 1993, 45, 509–511. [Google Scholar] [CrossRef]
- Strehlow, K.; Rotter, S.; Wassmann, S.; Adam, O.; Grohe, C.; Laufs, K.; Bohm, M.; Nickenig, G. Modulation of antioxidant enzyme expression and function by estrogen. Circ. Res. 2003, 93, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gou, Y.; Zhang, H.; Zuo, H.; Zhang, H.; Liu, Z.; Yao, D. Estradiol improves cardiovascular function through up-regulation of SOD2 on vascular wall. Redox Biol. 2014, 3, 88–99. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.H.; Schroeter, M.R.; Hecker, M. 17β-estradiol inhibition of NADPH oxidase expression in human endothelial cells. FASEB J. 2001, 15, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Gomez, X.; Novella, S.; Perez-Monzo, I.; Garabito, M.; Dantas, A.P.; Segarra, G.; Hermenegildo, C.; Medina, P. Decreased bioavailability of nitric oxide in aorta from ovariectomized senescent mice. Role of cyclooxygenase. Exp. Gerontol. 2016, 76, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.S.; Marsden, P.A. Epigenetics in the Vascular Endothelium: Looking From a Different Perspective in the Epigenomics Era. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Abi Khalil, C. The emerging role of epigenetics in cardiovascular disease. Ther. Adv. Chronic Dis. 2014, 5, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Voelter-Mahlknecht, S. Epigenetic associations in relation to cardiovascular prevention and therapeutics. Clin. Epigenetics 2016, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Post, W.S.; Goldschmidt-Clermont, P.J.; Wilhide, C.C.; Heldman, A.W.; Sussman, M.S.; Ouyang, P.; Milliken, E.E.; Issa, J.-P.J. Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc. Res. 1999, 43, 985–991. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.Y.; Song, K.S.; Lee, Y.H.; Seo, J.S.; Jelinek, J.; Goldschmidt-Clermont, P.J.; Issa, J.-P.J. Epigenetic changes in estrogen receptor β gene in atherosclerotic cardiovascular tissues and in-vitro vascular senescence. Biochim. Biophys. Acta 2007, 1772, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Kawagoe, J.; Ohmichi, M.; Tsutsumi, S.; Ohta, T.; Takahashi, K.; Kurachi, H. Mechanism of the Divergent Effects of Estrogen on the Cell Proliferation of Human Umbilical Endothelial Versus Aortic Smooth Muscle Cells. Endocrinology 2007, 148, 6092–6099. [Google Scholar] [CrossRef] [PubMed]
- Bendale, D.S.; Karpe, P.A.; Chhabra, R.; Shete, S.P.; Shah, H.; Tikoo, K. 17-β Oestradiol prevents cardiovascular dysfunction in post-menopausal metabolic syndrome by affecting SIRT1/AMPK/H3 acetylation. Br. J. Pharmacol. 2013, 170, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Kaikkonen, M.U.; Lam, M.T.; Glass, C.K. Non-coding RNAs as regulators of gene expression and epigenetics. Cardiovasc. Res. 2011, 90, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Hendrix, D.; Levine, M.; Haley, B. A distinct class of small RNAs arises from pre-miRNA-proximal regions in a simple chordate. Nat. Struct. Mol. Biol. 2009, 16, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Schnitzler, G.R.; Iyer, L.K.; Aronovitz, M.J.; Baur, W.E.; Karas, R.H. MicroRNA-Offset RNA Alters Gene Expression and Cell Proliferation. PLoS ONE 2016, 11, e0156772. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.N.; Han, J.; Siomi, M.C. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell Biol. 2009, 10, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Sobrino, A.; Mata, M.; Laguna-Fernandez, A.; Novella, S.; Oviedo, P.J.; Garcia-Perez, M.A.; Tarin, J.J.; Cano, A.; Hermenegildo, C. Estradiol stimulates vasodilatory and metabolic pathways in cultured human endothelial cells. PLoS ONE 2009, 4, e8242. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Fu, X.; Alves, P.; Gerstein, M. mRNA expression profiles show differential regulatory effects of microRNAs between estrogen receptor-positive and estrogen receptor-negative breast cancer. Genome Biol. 2009, 10, R90. [Google Scholar] [CrossRef] [PubMed]
- Cizeron-Clairac, G.; Lallemand, F.; Vacher, S.; Lidereau, R.; Bieche, I.; Callens, C. MiR-190b, the highest up-regulated miRNA in ERα-positive compared to ERα-negative breast tumors, a new biomarker in breast cancers? BMC Cancer 2015, 15, 499. [Google Scholar] [CrossRef] [PubMed]
- Bhat-Nakshatri, P.; Wang, G.; Collins, N.R.; Thomson, M.J.; Geistlinger, T.R.; Carroll, J.S.; Brown, M.; Hammond, S.; Srour, E.F.; Liu, Y.; et al. Estradiol-regulated microRNAs control estradiol response in breast cancer cells. Nucleic Acids Res. 2009, 37, 4850–4861. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, D.R.; Cittelly, D.M.; Howe, E.N.; Spoelstra, N.S.; McKinsey, E.L.; LaPara, K.; Elias, A.; Yee, D.; Richer, J.K. MicroRNAs link estrogen receptor α status and Dicer levels in breast cancer. Horm. Cancer 2010, 1, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Paris, O.; Ferraro, L.; Grober, O.M.; Ravo, M.; De Filippo, M.R.; Giurato, G.; Nassa, G.; Tarallo, R.; Cantarella, C.; Rizzo, F.; et al. Direct regulation of microRNA biogenesis and expression by estrogen receptor beta in hormone-responsive breast cancer. Oncogene 2012, 31, 4196–4206. [Google Scholar] [CrossRef] [PubMed]
- Nothnick, W.B.; Healy, C.; Hong, X. Steroidal regulation of uterine miRNAs is associated with modulation of the miRNA biogenesis components Exportin-5 and Dicer1. Endocrine 2010, 37, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Castellano, L.; Giamas, G.; Jacob, J.; Coombes, R.C.; Lucchesi, W.; Thiruchelvam, P.; Barton, G.; Jiao, L.R.; Wait, R.; Waxman, J.; et al. The estrogen receptor-α-induced microRNA signature regulates itself and its transcriptional response. Proc. Natl. Acad. Sci. USA 2009, 106, 15732–15737. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. miRNAs and estrogen action. Trends Endocrinol. Metab. 2012, 23, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Al-Nakhle, H.; Burns, P.A.; Cummings, M.; Hanby, A.M.; Hughes, T.A.; Satheesha, S.; Shaaban, A.M.; Smith, L.; Speirs, V. Estrogen receptor {β}1 expression is regulated by miR-92 in breast cancer. Cancer Res. 2010, 70, 4778–4784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, X.; Chen, Z.; Wang, W. MicroRNA-424 suppresses estradiol-induced cell proliferation via targeting GPER in endometrial cancer cells. Cell. Mol. Biol. 2015, 61, 96–101. [Google Scholar] [PubMed]
- Yang, W.J.; Yang, D.D.; Na, S.; Sandusky, G.E.; Zhang, Q.; Zhao, G. Dicer is required for embryonic angiogenesis during mouse development. J. Biol. Chem. 2005, 280, 9330–9335. [Google Scholar] [CrossRef] [PubMed]
- Suarez, Y.; Fernandez-Hernando, C.; Pober, J.S.; Sessa, W.C. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ. Res. 2007, 100, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Kuehbacher, A.; Urbich, C.; Zeiher, A.M.; Dimmeler, S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ. Res. 2007, 101, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Eghbali, M. Influence of sex differences on microRNA gene regulation in disease. Biol. Sex Differ. 2014, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhang, Q.; Ma, X.; Wang, J.; Liang, T. miRNA and mRNA expression analysis reveals potential sex-biased miRNA expression. Sci. Rep. 2017, 7, 39812. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. Estrogen action: Receptors, transcripts, cell signaling, and non-coding RNAs in normal physiology and disease. Mol. Cell. Endocrinol. 2015, 418, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, K.; Pang, Q.; Yang, C.; Zhang, H.; Wu, F.; Cao, H.; Liu, H.; Wan, Y.; Xia, W.; et al. Identification of suitable reference gene and biomarkers of serum miRNAs for osteoporosis. Sci. Rep. 2016, 6, 36347. [Google Scholar] [CrossRef] [PubMed]
- Kangas, R.; Tormakangas, T.; Fey, V.; Pursiheimo, J.; Miinalainen, I.; Alen, M.; Kaprio, J.; Sipila, S.; Saamanen, A.M.; Kovanen, V.; et al. Aging and serum exomiR content in women-effects of estrogenic hormone replacement therapy. Sci. Rep. 2017, 7, 42702. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.M.; Deschamps, A.M.; Liu, D.; Raghavachari, N.; Murphy, E. miR-222 contributes to sex-dimorphic cardiac eNOS expression via ets-1. Physiol. Genom. 2013, 45, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Queirós, A.M.; Eschen, C.; Fliegner, D.; Kararigas, G.; Dworatzek, E.; Westphal, C.; Sanchez Ruderisch, H.; Regitz-Zagrosek, V. Sex- and estrogen-dependent regulation of a miRNA network in the healthy and hypertrophied heart. Int. J. Cardiol. 2013, 169, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Petrov, G.; Regitz-Zagrosek, V.; Lehmkuhl, E.; Krabatsch, T.; Dunkel, A.; Dandel, M.; Dworatzek, E.; Mahmoodzadeh, S.; Schubert, C.; Becher, E.; et al. Regression of Myocardial Hypertrophy After Aortic Valve Replacement. Circulation 2010, 122, S23. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Sun, L.-Y.; Zhang, S.-C.; Wei, R.; Xie, F.; Liu, J.; Yan, Y.; Duan, M.-J.; Sun, L.-L.; Sun, Y.-H.; et al. MicroRNA-23a Participates in Estrogen Deficiency Induced Gap Junction Remodeling of Rats by Targeting GJA1. Int. J. Biol. Sci. 2015, 11, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Gowd, B.M.P.; Thompson, P.D. Effect of Female Sex on Cardiac Arrhythmias. Cardiol. Rev. 2012, 20, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Siegel, C.; Li, J.; Liu, F.; Benashski, S.E.; McCullough, L.D. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proc. Natl. Acad. Sci. USA 2011, 108, 11662–11667. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Moser, H.; McCullough, L.D. Sex differences in ischaemic stroke: Potential cellular mechanisms. Clin. Sci. 2017, 131, 533. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, Z.P.; Zhao, W.; Cong, B.H.; Lu, J.Q.; Tang, X.L.; Li, X.H.; Zhu, X.Y.; Ni, X. MiR-22/Sp-1 Links Estrogens With the Up-Regulation of Cystathionine gamma-Lyase in Myocardium, Which Contributes to Estrogenic Cardioprotection Against Oxidative Stress. Endocrinology 2015, 156, 2124–2137. [Google Scholar] [CrossRef] [PubMed]
- Schweisgut, J.; Schutt, C.; Wust, S.; Wietelmann, A.; Ghesquiere, B.; Carmeliet, P.; Drose, S.; Korach, K.S.; Braun, T.; Boettger, T. Sex-specific, reciprocal regulation of ERα and miR-22 controls muscle lipid metabolism in male mice. EMBO J. 2017, 36, 1199–1214. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Imbrie, G.A.; Baur, W.E.; Iyer, L.K.; Aronovitz, M.; Kershaw, T.B.; Haselmann, G.M.; Lu, Q.; Karas, R.H. Estrogen receptor-mediated regulation of microRNA inhibits proliferation of vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Blanco, F.J.; Stevens, H.; Lu, R.; Caudrillier, A.; McBride, M.; McClure, J.D.; Grant, J.; Thomas, M.; Frid, M.; et al. MicroRNA-143 Activation Regulates Smooth Muscle and Endothelial Cell Crosstalk in Pulmonary Arterial Hypertension. Circ. Res. 2015, 117, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Gomez, X.; Pérez-Cremades, D.; Mompeón, A.; Dantas, A.P.; Novella, S.; Hermenegildo, C. microRNA as crucial regulators of gene expression in estradiol-treated human endothelial cells. Cell. Physiol. Biochem. 2018, in press. [Google Scholar]
- Li, P.; Wei, J.; Li, X.; Cheng, Y.; Chen, W.; Cui, Y.; Simoncini, T.; Gu, Z.; Yang, J.; Fu, X. 17β-estradiol enhances vascular endothelial Ets-1/miR-126-3p expression: The possible mechanism for attenuation of atherosclerosis. J. Clin. Endocrinol. Metab. 2016, 102, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D. Sex Differences in Inflammation during Atherosclerosis. Clin. Med. Insights Cardiol. 2014, 8 (Suppl. 3), 49–59. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.J.; Guyre, P.M.; Pioli, P.A. Estradiol suppresses NF-κB activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J. Immunol. 2010, 184, 5029–5037. [Google Scholar] [CrossRef] [PubMed]
- Dai, R.; Phillips, R.A.; Zhang, Y.; Khan, D.; Crasta, O.; Ahmed, S.A. Suppression of LPS-induced Interferon-γ and nitric oxide in splenic lymphocytes by select estrogen-regulated microRNAs: A novel mechanism of immune modulation. Blood 2008, 112, 4591–4597. [Google Scholar] [CrossRef] [PubMed]
- Olivieri, F.; Ahtiainen, M.; Lazzarini, R.; Pöllänen, E.; Capri, M.; Lorenzi, M.; Fulgenzi, G.; Albertini, M.C.; Salvioli, S.; Alen, M.J.; et al. Hormone replacement therapy enhances IGF-1 signaling in skeletal muscle by diminishing miR-182 and miR-223 expressions: A study on postmenopausal monozygotic twin pairs. Aging Cell 2014, 13, 850–861. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Ohn, J.H.; Song, J.A.; Yang, J.-Y.; Park, H.; Choi, H.J.; Kim, S.W.; Kim, S.Y.; Park, W.-Y.; Shin, C.S. Changes of MicroRNA Profile and MicroRNA-mRNA Regulatory Network in Bones of Ovariectomized Mice. J. Bone Miner. Res. 2014, 29, 644–656. [Google Scholar] [CrossRef] [PubMed]
- Richelson, L.S.; Wahner, H.W.; Melton, L.J.I.; Riggs, B.L. Relative Contributions of Aging and Estrogen Deficiency to Postmenopausal Bone Loss. N. Engl. J. Med. 1984, 311, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Kangas, R.; Pollanen, E.; Rippo, M.R.; Lanzarini, C.; Prattichizzo, F.; Niskala, P.; Jylhava, J.; Sipila, S.; Kaprio, J.; Procopio, A.D.; et al. Circulating miR-21, miR-146a and Fas ligand respond to postmenopausal estrogen-based hormone replacement therapy-a study with monozygotic twin pairs. Mech. Ageing Dev. 2014, 143–144, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Goedeke, L.; Rotllan, N.; Canfrán-Duque, A.; Aranda, J.F.; Ramírez, C.M.; Araldi, E.; Lin, C.-S.; Anderson, N.N.; Wagschal, A.; de Cabo, R.; et al. Identification of miR-148a as a novel regulator of cholesterol metabolism. Nat. Med. 2015, 21, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Windler, E.E.; Kovanen, P.T.; Chao, Y.S.; Brown, M.S.; Havel, R.J.; Goldstein, J.L. The estradiol-stimulated lipoprotein receptor of rat liver. A binding site that membrane mediates the uptake of rat lipoproteins containing apoproteins B and E. J. Biol. Chem. 1980, 255, 10464–10471. [Google Scholar] [PubMed]
- Goedeke, L.; Rotllan, N.; Ramírez, C.M.; Aranda, J.F.; Canfrán-Duque, A.; Araldi, E.; Fernández-Hernando, A.; Langhi, C.; de Cabo, R.; Baldán, Á.; et al. miR-27b inhibits LDLR and ABCA1 expression but does not influence plasma and hepatic lipid levels in mice. Atherosclerosis 2015, 243, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Dolz, S.; Górriz, D.; Tembl, J.I.; Sánchez, D.; Fortea, G.; Parkhutik, V.; Lago, A. Circulating MicroRNAs as Novel Biomarkers of Stenosis Progression in Asymptomatic Carotid Stenosis. Stroke 2016, 48, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Buratti, L.; Balestrini, S.; Avitabile, E.; Altamura, C.; Vernieri, F.; Viticchi, G.; Falsetti, L.; Provinciali, L.; Silvestrini, M. Sex-associated differences in the modulation of vascular risk in patients with asymptomatic carotid stenosis. J. Cereb. Blood Flow Metab. 2015, 35, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.-F.; Chen, H.; Song, J.-X. MiR-106b-5p Inhibits Tumor Necrosis Factor-induced Apoptosis by Targeting Phosphatase and Tensin Homolog Deleted on Chromosome 10 in Vascular Endothelial Cells. Chin. Med. J. 2016, 129, 1406–1412. [Google Scholar] [PubMed]
- Noh, E.M.; Lee, Y.R.; Chay, K.O.; Chung, E.Y.; Jung, S.H.; Kim, J.S.; Youn, H.J. Estrogen receptor α induces down-regulation of PTEN through PI3-kinase activation in breast cancer cells. Mol. Med. Rep. 2011, 4, 215–219. [Google Scholar] [PubMed]
- Smith, J.A.; Zhang, R.; Varma, A.K.; Das, A.; Ray, S.K.; Banik, N.L. Estrogen partially down-regulates PTEN to prevent apoptosis in VSC4.1 motoneurons following exposure to IFN-γ. Brain Res. 2009, 1301 (Suppl. C), 163–170. [Google Scholar] [CrossRef] [PubMed]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.F.; Wythe, J.D.; Ivey, K.N.; Bruneau, B.G.; Stainier, D.Y.; Srivastava, D. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell 2008, 15, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell 2008, 15, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.A.; Yamakuchi, M.; Ferlito, M.; Mendell, J.T.; Lowenstein, C.J. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. USA 2008, 105, 1516–1521. [Google Scholar] [CrossRef] [PubMed]

| Probe Set ID | Symbol | Official Full Name | Fold Change | p Value |
|---|---|---|---|---|
| 218269_at | DROSHA | drosha, ribonuclease type III | −1.117 | 0.586 |
| 64474_g_at | DGCR8 | DiGeorge syndrome critical region gene 8 | 2.376 | 0.016 |
| 223056_s_at | XPO5 | exportin 5 | 1.514 | 0.259 |
| 213229_at | DICER1 | dicer 1, ribonuclease type III | −1.979 | 0.012 |
| 225569_at | AGO-2 | argonaute-2 | −1.290 | 0.002 |
| Estrogen Action | miRNA | References |
|---|---|---|
| Sex differences in heart | miR-1 miR-106b miR-720 miR-29b miR-144 miR-34b-5p miR-205 miR-222 | [95] |
| Sex differences in cardiac fibrosis | miR-21 miR-24 miR-27a/b miR-106a/b | [96] |
| Cardiac gap junction regulation | miR-23a | [98] |
| Regulation of oxidative stress in the myocardium | miR-22 | [102] |
| Inhibition of VSMC proliferation | miR-203 | [104] |
| VSMC and endothelial cell communication | miR-143 miR-145 | [105] |
| Endothelial cell proliferation | miR-126-3p | [107] |
| miRNA expression profile in estradiol-treated endothelial cells | miR-30b-5p miR487a-5p miR-4710 miR-501-3p miR-378h miR-1244 | [106] |
| Regulation of NF-kB pathway in macrophages | let-7a and miR-125b | [109] |
| Regulation of IFNγ released in lymphocytes | miR-146a miR-223 | [110] |
| Regulation of Insulin/IGF-1 pathway in skeletal muscle | miR-182 and miR-223 | [111] |
| Circulating Inflammation markers | miR-21 miR-146a | [105] |
| Negative regulation of bone mass. | miR-127 and miR-136 | [112] |
| Serum biomarker in osteoporosis | miR-30b-5p | [93] |
| Circulating miRNA | miR-106-5p miR-148a-3p miR-27-3p miR-126-5p miR-28-3p miR-30a-5p | [94] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Cremades, D.; Mompeón, A.; Vidal-Gómez, X.; Hermenegildo, C.; Novella, S. miRNA as a New Regulatory Mechanism of Estrogen Vascular Action. Int. J. Mol. Sci. 2018, 19, 473. https://doi.org/10.3390/ijms19020473
Pérez-Cremades D, Mompeón A, Vidal-Gómez X, Hermenegildo C, Novella S. miRNA as a New Regulatory Mechanism of Estrogen Vascular Action. International Journal of Molecular Sciences. 2018; 19(2):473. https://doi.org/10.3390/ijms19020473
Chicago/Turabian StylePérez-Cremades, Daniel, Ana Mompeón, Xavier Vidal-Gómez, Carlos Hermenegildo, and Susana Novella. 2018. "miRNA as a New Regulatory Mechanism of Estrogen Vascular Action" International Journal of Molecular Sciences 19, no. 2: 473. https://doi.org/10.3390/ijms19020473
APA StylePérez-Cremades, D., Mompeón, A., Vidal-Gómez, X., Hermenegildo, C., & Novella, S. (2018). miRNA as a New Regulatory Mechanism of Estrogen Vascular Action. International Journal of Molecular Sciences, 19(2), 473. https://doi.org/10.3390/ijms19020473