Nano-Pore Size of Alumina Affects Osteoblastic Response
Abstract
:1. Introduction
2. Results
2.1. Morphology and Elemental Composition
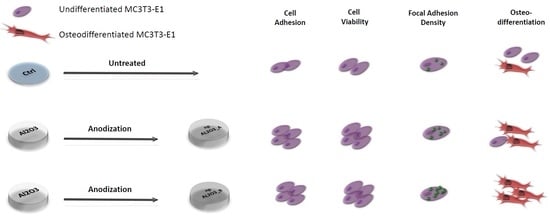
2.2. Cell Adhesion
2.3 Cell Viability
2.4. Cell Morphology and Focal Adhesion Quantification
2.5. Osteogenic Differentiation
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy
4.3. Biological Assays
4.3.1. Cell Adhesion
4.3.2. Cell Viability
4.3.3. Cell Morphology and Focal Adhesion Quantification
4.3.4. Osteogenic Cell Differentiation
4.3.5. Alkaline Phosphatase Activity
4.3.6. Calcium Content
4.4. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Al | Aluminum metal |
| AAO | Anodic Aluminum Oxide |
| FESEM | Field Emission Scanning Electron Microscope |
| EDX | Energy Dispersive X-ray spectroscopy |
| PBS | Phosphate Buffer Saline |
| ALP | Alkaline Phosphatase Activity |
References
- Albrektsson, T.; Sennerby, L. State of the art in oral implants. J. Clin. Periodontol. 1991, 18, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, L.W.; Carlsson, G.E.; Jemt, T. A prospective 15-year follow-up study of mandibular fixed prostheses supported by osseointegrated implants. Clinical results and marginal bone loss. Clin. Oral Implants Res. 1996, 7, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Evrard, L.; Waroquier, D.; Parent, D. Allergies to dental metals. Titanium: A new allergen. Rev. Med. Brux. 2010, 31, 44–49. [Google Scholar] [PubMed]
- Pigatto, P.D.; Guzzi, G.; Brambilla, L.; Sforza, C. Titanium allergy associated with dental implant failure: Letters to the Editor. Clin. Oral Implants Res. 2009, 20, 857. [Google Scholar] [CrossRef] [PubMed]
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium allergy in dental implant patients: A clinical study on 1500 consecutive patients. Clin. Oral Implants Res. 2008, 19, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Onodera, K.; Ooya, K.; Kawamura, H. Titanium lymph node pigmentation in the reconstruction plate system of a mandibular bone defect. Oral Surg. Oral Med. Oral Pathol. 1993, 75, 495–497. [Google Scholar] [CrossRef]
- Depprich, R.; Naujoks, C.; Ommerborn, M.; Schwarz, F.; Kübler, N.R.; Handschel, J. Current findings regarding zirconia implants. Clin. Implant Dent. Relat. Res. 2014, 16, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Andreiotelli, M.; Wenz, H.J.; Kohal, R.J. Are ceramic implants a viable alternative to titanium implants? A systematic literature review. Clin. Oral Implants Res. 2009, 20, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Spies, B.C.; Sperlich, M.; Fleiner, J.; Stampf, S.; Kohal, R.J. Alumina reinforced zirconia implants: 1-year results from a prospective cohort investigation. Clin. Oral Implants Res. 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schierano, G.; Mussano, F.; Faga, M.G.; Menicucci, G.; Manzella, C.; Sabione, C.; Genova, T.; von Degerfeld, M.M.; Peirone, B.; Cassenti, A.; et al. An Alumina Toughened Zirconia Composite for Dental Implant Application: In Vivo Animal Results. Biomed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cionca, N.; Hashim, D.; Mombelli, A. Zirconia dental implants: Where are we now, and where are we heading? Periodontology 2000 2017, 73, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Duraccio, D.; Mussano, F.; Faga, M.G. Biomaterials for dental implants: Current and future trends. J. Mater. Sci. 2015, 50, 4779–4812. [Google Scholar] [CrossRef]
- Mandracci, P.; Mussano, F.; Rivolo, P.; Carossa, S. Surface Treatments and Functional Coatings for Biocompatibility Improvement and Bacterial Adhesion Reduction in Dental Implantology. Coatings 2016, 6, 7. [Google Scholar] [CrossRef]
- Vallée, A.; Faga, M.G.; Mussano, F.; Catalano, F.; Tolosano, E.; Carossa, S.; Altruda, F.; Martra, G. Alumina-zirconia composites functionalized with laminin-1 and laminin-5 for dentistry: Effect of protein adsorption on cellular response. Colloids Surf. B Biointerfaces 2014, 114, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Genova, T.; Laurenti, M.; Munaron, L.; Pirri, C.F.; Rivolo, P.; Carossa, S.; Mandracci, P. Hydrogenated amorphous silicon coatings may modulate gingival cell response. Appl. Surf. Sci. 2018, 436, 603–612. [Google Scholar] [CrossRef]
- Mussano, F.; Genova, T.; Verga Falzacappa, E.; Scopece, P.; Munaron, L.; Rivolo, P.; Mandracci, P.; Benedetti, A.; Carossa, S.; Patelli, A. In vitro characterization of two different atmospheric plasma jet chemical functionalizations of titanium surfaces. Appl. Surf. Sci. 2017, 409, 314–324. [Google Scholar] [CrossRef]
- Mandracci, P.; Mussano, F.; Ceruti, P.; Pirri, C.F.; Carossa, S. Reduction of bacterial adhesion on dental composite resins by silicon–oxygen thin film coatings. Biomed. Mater. 2015, 10, 15017. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Genova, T.; Rivolo, P.; Mandracci, P.; Munaron, L.; Faga, M.G.; Carossa, S. Role of surface finishing on the in vitro biological properties of a silicon nitride–titanium nitride (Si3N4–TiN) composite. J. Mater. Sci. 2017, 52, 467–477. [Google Scholar] [CrossRef]
- Gazia, R.; Mandracci, P.; Mussano, F.; Carossa, S. AlNx and a-SiOx coatings with corrosion resistance properties for dental implants. Surf. Coat. Technol. 2011, 206, 1109–1115. [Google Scholar] [CrossRef]
- Mandracci, P.; Ceruti, P.; Ricciardi, C.; Mussano, F.; Carossa, S. a-SiOX Coatings Grown on Dental Materials by PECVD: Compositional Analysis and Preliminary Investigation of Biocompatibility Improvements. Chem. Vap. Depos. 2010, 16, 29–34. [Google Scholar] [CrossRef]
- Mandracci, P.; Mussano, F.; Ricciardi, C.; Ceruti, P.; Pirri, F.; Carossa, S. Low temperature growth of thin film coatings for the surface modification of dental prostheses. Surf. Coat. Technol. 2008, 202, 2477–2481. [Google Scholar] [CrossRef]
- Mendes, P.M. Cellular nanotechnology: Making biological interfaces smarter. Chem. Soc. Rev. 2013, 42, 9207. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implants Res. 2009, 20, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.; Bornstein, M.M.; Wittneben, J.-G.; Martin, W.C.; Ruskin, J.D.; Hart, C.N.; Buser, D. Early Loading after 21 Days of Healing of Nonsubmerged Titanium Implants with a Chemically Modified Sandblasted and Acid-Etched Surface: Two-Year Results of a Prospective Two-Center Study. Clin. Implant Dent. Relat. Res. 2010, 12, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.M.; Schmid, B.; Belser, U.C.; Lussi, A.; Buser, D. Early loading of non-submerged titanium implants with a sandblasted and acid-etched surface. Clin. Oral Implants Res. 2005, 16, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O.C. Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.L.; Kato, R.B.; Castro Raucci, L.M.S.; Teixeira, L.N.; de Oliveira, F.S.; Bellesini, L.S.; de Oliveira, P.T.; Hassan, M.Q.; Beloti, M.M. Nanotopography Drives Stem Cell Fate Toward Osteoblast Differentiation Through α1β1 Integrin Signaling Pathway. J. Cell. Biochem. 2014, 115, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Puckett, S.; Pareta, R.; Webster, T.J. Nano rough micron patterned titanium for directing osteoblast morphology and adhesion. Int. J. Nanomed. 2008, 3, 229–241. [Google Scholar] [CrossRef]
- Srivatsan, T.S. Biomaterials: A Nano Approach, by Sreeram Ramakrishna, Murugan Ramalingam, T.S. Sampath Kumar, and Winston O. Soboyejo. Mater. Manuf. Process. 2014, 29, 1510–1511. [Google Scholar] [CrossRef]
- Greco, R.S.; Prinz, F.B.; Smith, R.L. Nanoscale Technology in Biological Systems; CRC Press: Boca Raton, FL, USA, 2005; p. 485. [Google Scholar]
- Aguilar, Z.P. Nanomaterials for Medical Applications; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 9780123850904. [Google Scholar]
- DeMali, K.A.; Wennerberg, K.; Burridge, K. Integrin signaling to the actin cytoskeleton. Curr. Opin. Cell Biol. 2003, 15, 572–582. [Google Scholar] [CrossRef]
- Hoffmann, B.; Schäfer, C. Filopodial focal complexes direct adhesion and force generation towards filopodia outgrowth. Cell Adhes. Migr. 2010, 4, 190–193. [Google Scholar] [CrossRef]
- Gallagher, J.O.; McGhee, K.F.; Wilkinson, C.D.W.; Riehle, M.O. Interaction of animal cells with ordered nanotopography. IEEE Trans. Nanobiosci. 2002, 1, 24–28. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Ali, N.; Fawcett, D. Progress in Nano-Engineered Anodic Aluminum Oxide Membrane Development. Materials 2011, 4, 487–526. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Yada, K.; Osaka, A. Self-Ordering of Cell Configuration of Anodic Porous Alumina with Large-Size Pores in Phosphoric Acid Solution. Jpn. J. Appl. Phys. 1998, 37, L1340–L1342. [Google Scholar] [CrossRef]
- Song, Y.; Ju, Y.; Morita, Y.; Xu, B.; Song, G. Surface functionalization of nanoporous alumina with bone morphogenetic protein 2 for inducing osteogenic differentiation of mesenchymal stem cells. Mater. Sci. Eng. C 2014, 37, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Pålsgård, E.; Wilshaw, P.; Di Silvio, L. Initial in vitro interaction of osteoblasts with nano-porous alumina. Biomaterials 2003, 24, 3039–3046. [Google Scholar] [CrossRef]
- Swan, E.E.L.; Popat, K.C.; Grimes, C.A.; Desai, T.A. Fabrication and evaluation of nanoporous alumina membranes for osteoblast culture. J. Biomed. Mater. Res. Part A 2005, 72A, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ju, Y.; Morita, Y.; Song, G. Effect of the nanostructure of porous alumina on growth behavior of MG63 osteoblast-like cells. J. Biosci. Bioeng. 2013, 116, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Li, C.; Ni, S.; Chen, T.; Webster, T.J. Understanding improved osteoblast behavior on select nanoporous anodic alumina. Int. J. Nanomed. 2014, 9, 3325–3334. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ju, Y.; Song, G.; Morita, Y. In vitro proliferation and osteogenic differentiation of mesenchymal stem cells on nanoporous alumina. Int. J. Nanomed. 2013, 8, 2745. [Google Scholar] [CrossRef]
- Genova, T.; Munaron, L.; Carossa, S.; Mussano, F. Overcoming physical constraints in bone engineering: The importance of being vascularized. J. Biomater. Appl. 2016, 30, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Riehle, M.O.; Johnstone, H.; Affrossman, S.; Curtis, A.S.G. In vitro reaction of endothelial cells to polymer demixed nanotopography. Biomaterials 2002, 23, 2945–2954. [Google Scholar] [CrossRef]
- Guadarrama Bello, D.; Fouillen, A.; Badia, A.; Nanci, A. A nanoporous titanium surface promotes the maturation of focal adhesions and formation of filopodia with distinctive nanoscale protrusions by osteogenic cells. Acta Biomater. 2017, 60, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, J.; Sim, S.H.; Sung, M.G.; Kim, K.S.; Hong, B.H.; Hong, S. Enhanced Differentiation of Human Neural Stem Cells into Neurons on Graphene. Adv. Mater. 2011, 23, H263–H267. [Google Scholar] [CrossRef] [PubMed]
- McMurray, R.J.; Gadegaard, N.; Tsimbouri, P.M.; Burgess, K.V.; McNamara, L.E.; Tare, R.; Murawski, K.; Kingham, E.; Oreffo, R.O.C.; Dalby, M.J. Nanoscale surfaces for the long-term maintenance of mesenchymal stem cell phenotype and multipotency. Nat. Mater. 2011, 10, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Choi, D.S.; Jin, H.J.; Park, J.; Byun, K.-E.; Lee, K.-B.; Hong, S. Polarization-Controlled Differentiation of Human Neural Stem Cells Using Synergistic Cues from the Patterns of Carbon Nanotube Monolayer Coating. ACS Nano 2011, 5, 4704–4711. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.-H.; Bernard, C.; Variola, F.; Zalzal, S.F.; Wuest, J.D.; Rosei, F.; Nanci, A. Characterization of a bioactive nanotextured surface created by controlled chemical oxidation of titanium. Surf. Sci. 2006, 600, 4613–4621. [Google Scholar] [CrossRef]
- De Oliveira, P.T.; Nanci, A. Nanotexturing of titanium-based surfaces upregulates expression of bone sialoprotein and osteopontin by cultured osteogenic cells. Biomaterials 2004, 25, 403–413. [Google Scholar] [CrossRef]
- Nune, K.; Misra, R.; Gai, X.; Li, S.; Hao, Y. Surface nanotopography-induced favorable modulation of bioactivity and osteoconductive potential of anodized 3D printed Ti-6Al-4V alloy mesh structure. J. Biomater. Appl. 2017, 88532821774886. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.G.; De Oliveira, P.T.; Nanci, A.; Hawthorne, A.C.; Rosa, A.L.; Xavier, S.P. Treatment of a commercial, machined surface titanium implant with H2SO4/H2O2 enhances contact osteogenesis. Clin. Oral Implants Res. 2007, 18, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Lavenus, S.; Berreur, M.; Trichet, V.; Pilet, P.; Louarn, G.; Layrolle, P. Adhesion and osteogenic differentiation of human mesenchymal stem cells on titanium nanopores. Eur. Cells Mater. 2011, 22, 84–96. [Google Scholar] [CrossRef]
- Nasrollahi, S.; Banerjee, S.; Qayum, B.; Banerjee, P.; Pathak, A. Nanoscale Matrix Topography Influences Microscale Cell Motility through Adhesions, Actin Organization, and Cell Shape. ACS Biomater. Sci. Eng. 2017, 3, 2980–2986. [Google Scholar] [CrossRef]
- Chen, Z.; Ni, S.; Han, S.; Crawford, R.; Lu, S.; Wei, F.; Chang, J.; Wu, C.; Xiao, Y. Nanoporous microstructures mediate osteogenesis by modulating the osteo-immune response of macrophages. Nanoscale 2017, 9, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Genova, T.; Tallarico, M.; Gautier, G.; Mussano, F.; Botticelli, D. Plasma of Argon Affects the Earliest Biological Response of Different Implant Surfaces. J. Dent. Res. 2016, 95, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Genova, T.; Mandracci, P.; Mussano, F.; Abundo, R.; Fiorellini, J. Morphometric Changes Induced by Cold Argon Plasma Treatment on Osteoblasts Grown on Different Dental Implant Surfaces. Int. J. Periodontics Restor. Dent. 2017, 37, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Genova, T.; Wang, H.-L.; Carossa, S.; Mussano, F. Plasma of Argon Increases Cell Attachment and Bacterial Decontamination on Different Implant Surfaces. Int. J. Oral Maxillofac. Implants 2017, 32, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Genova, T.; Grolez, G.P.; Camillo, C.; Bernardini, M.; Bokhobza, A.; Richard, E.; Scianna, M.; Lemonnier, L.; Valdembri, D.; Munaron, L.; et al. TRPM8 inhibits endothelial cell migration via a nonchannel function by trapping the small GTPase Rap1. J. Cell Biol. 2017, 216. [Google Scholar] [CrossRef] [PubMed]
- Avanzato, D.; Genova, T.; Fiorio Pla, A.; Bernardini, M.; Bianco, S.; Bussolati, B.; Mancardi, D.; Giraudo, E.; Maione, F.; Cassoni, P.; et al. Activation of P2X7 and P2Y11 purinergic receptors inhibits migration and normalizes tumor-derived endothelial cells via cAMP signaling. Sci. Rep. 2016, 6, 32602. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Bartorelli Cusani, A.; Brossa, A.; Carossa, S.; Bussolati, G.; Bussolati, B. Presence of osteoinductive factors in bovine colostrum. Biosci. Biotechnol. Biochem. 2014, 78, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Lee, K.J.; Zuk, P.; Tran, L.; Cacalano, N.A.; Jewett, A.; Carossa, S.; Nishimura, I. Differential effect of ionizing radiation exposure on multipotent and differentiation-restricted bone marrow mesenchymal stem cells. J. Cell. Biochem. 2010, 111, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Genova, T.; Corsalini, M.; Schierano, G.; Pettini, F.; Di Venere, D.; Carossa, S. Cytokine, Chemokine, and Growth Factor Profile Characterization of Undifferentiated and Osteoinduced Human Adipose-Derived Stem Cells. Stem Cells Int. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]







| Surface Features | Sample A | Sample B | |
|---|---|---|---|
| Pore distribution | Homogenous | Homogenous | |
| Pore diameter on the surface | 16–30 nm | 65–89 nm | |
| Pore diameter in the cross-section of the sample | Surface | 16–30 nm | 64–87 nm |
| Center | 16–20 nm | 47–62 nm | |
| Substrate | 10–20 nm | 25–40 nm | |
| Thickness | 25 µm | 82 µm | |
| Element | wt % | at % |
|---|---|---|
| C | 39.39 | 55.78 |
| O | 20.38 | 21.26 |
| Al | 35.06 | 22.10 |
| Pt | 5.17 | 0.45 |
| Total | 100.00 | 100.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mussano, F.; Genova, T.; Serra, F.G.; Carossa, M.; Munaron, L.; Carossa, S. Nano-Pore Size of Alumina Affects Osteoblastic Response. Int. J. Mol. Sci. 2018, 19, 528. https://doi.org/10.3390/ijms19020528
Mussano F, Genova T, Serra FG, Carossa M, Munaron L, Carossa S. Nano-Pore Size of Alumina Affects Osteoblastic Response. International Journal of Molecular Sciences. 2018; 19(2):528. https://doi.org/10.3390/ijms19020528
Chicago/Turabian StyleMussano, Federico, Tullio Genova, Francesca Giulia Serra, Massimo Carossa, Luca Munaron, and Stefano Carossa. 2018. "Nano-Pore Size of Alumina Affects Osteoblastic Response" International Journal of Molecular Sciences 19, no. 2: 528. https://doi.org/10.3390/ijms19020528
APA StyleMussano, F., Genova, T., Serra, F. G., Carossa, M., Munaron, L., & Carossa, S. (2018). Nano-Pore Size of Alumina Affects Osteoblastic Response. International Journal of Molecular Sciences, 19(2), 528. https://doi.org/10.3390/ijms19020528