The Role of Oral Cavity Biofilm on Metallic Biomaterial Surface Destruction–Corrosion and Friction Aspects
Abstract
:1. Introduction
2. Microbial Flora and Biofilm Formation in Oral Environments
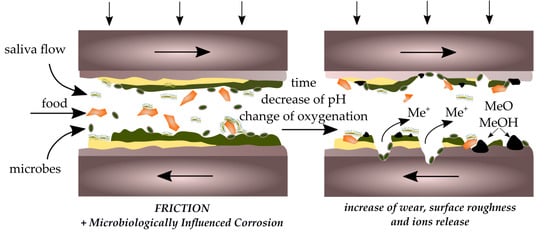
3. Corrosion
4. Biocorrosion
AMP + SO32− (sulfite reductase) → H2S
5. Friction and Wear
6. The Role of Saliva in Biofilm Growth
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fathi, M.H.; Salehi, M.; Saatchi, A.; Mortazavi, V.; Moosavi, S.B. In vitro corrosion behavior of bioceramic, metallic, and bioceramic-metallic coated stainless steel dental implants. Dent. Mater. 2003, 19, 188–198. [Google Scholar] [CrossRef]
- Ganzorig, K.; Kuroda, S.; Maeda, Y.; Mansjur, K.; Sato, M.; Nagata, K.; Tanaka, E. Low-intensity pulsed ultrasound enhances bone formation around miniscrew implants. Arch. Oral Biol. 2015, 60, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lakes, R.S. Biomaterials: An introduction, 3rd ed.; Springer: New York, NY, USA, 2007. [Google Scholar]
- Sharifnabi, A.; Fathi, M.H.; Eftekhari Yekta, B.; Hossainalipour, M. The structural and bio-corrosion barrier performance of Mg-substituted fluorapatite coating on 316l stainless steel human body implant. Appl. Surf. Sci. 2014, 288, 331–340. [Google Scholar] [CrossRef]
- Hsu, R.W.W.; Yang, C.C.; Huang, C.A.; Chen, Y.S. Electrochemical corrosion studies on Co-Cr-Mo implant alloy in biological solutions. Mater. Chem. Phys. 2005, 93, 531–538. [Google Scholar] [CrossRef]
- Kuphasuk, C.; Oshida, Y.; Andres, C.J.; Hovijitra, S.T.; Barco, M.T.; Brown, D.T. Electrochemical corrosion of titanium and titanium-based alloys. J. Prosthet. Dent. 2001, 85, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, T.P.; Upadhayay, S.N. An overview of orthodontic material degradation in oral cavity. Indian J. Dent. Res. 2010, 21, 275. [Google Scholar] [CrossRef] [PubMed]
- Mystkowska, J.; Karalus, W.; Sidorenko, J.; Dabrowski, J.R.; Kalska-Szostko, B. Biotribological properties of dentures lubricated with artificial saliva. J. Frict. Wear 2016, 37, 544–551. [Google Scholar] [CrossRef]
- Van Noort, R. Introduction to Dental Materials, 4th ed.; Elsevier Health Sciences: London, UK, 2013. [Google Scholar]
- Schipper, R.G.; Silletti, E.; Vingerhoeds, M.H. Saliva as research material: Biochemical, physicochemical and practical aspects. Arch. Oral Biol. 2007, 52, 1114–1135. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C.; Pedersen, A.M.L.; Villa, A.; Ekstrom, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the world workshop on oral medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, D.; Yakubov, G.E.; Stokes, J.R.; Williamson, A.M.; Fuller, G.G. Interaction of human whole saliva and astringent dietary compounds investigated by interfacial shear rheology. Food Hydrocoll. 2008, 22, 1068–1078. [Google Scholar] [CrossRef]
- Amerongen, A.V.N.; Veerman, E.C.I. Saliva—The defender of the oral cavity. Oral Dis. 2002, 8, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Corfield, A.P. Mucins: A biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta 2015, 1850, 236–252. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Lewandowski, Z. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Chand, D.V.; Chandra, J.; Anderson, J.M.; Ghannoum, M.A. Shear stress modulates the thickness and architecture of Candida albicans biofilms in a phase-dependent manner. Mycoses 2009, 52, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.C.M.; Henriques, M.; Oliveira, R.; Teughels, W.; Celis, J.P.; Rocha, L.A. Biofilms inducing ultra-low friction on titanium. J. Dent. Res. 2010, 89, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.C.M.; Henriques, M.; Oliveira, R.; Teughels, W.; Celis, J.P.; Rocha, L.A. Do oral biofilms influence the wear and corrosion behavior of titanium? Biofouling 2010, 26, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.C.M.; Barbosa, S.L.; Ariza, E.; Celis, J.P.; Rocha, L.A. Simultaneous degradation by corrosion and wear of titanium in artificial saliva containing fluorides. Wear 2012, 292–293, 82–88. [Google Scholar] [CrossRef]
- Apaza-Bedoya, K.; Tarce, M.; Benfatti, C.A.M.; Henriques, B.; Mathew, M.T.; Teughels, W.; Souza, J.C.M. Synergistic interactions between corrosion and wear at titanium-based dental implant connections: A scoping review. J. Periodontal Res. 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Stimmelmayr, M.; Edelhoff, D.; Güth, J.F.; Erdelt, K.; Happe, A.; Beuer, F. Wear at the titanium-titanium and the titanium-zirconia implant-abutment interface: A comparative in vitro study. Dent. Mater. 2012, 28, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Harada, R.; Takemoto, S.; Kinoshita, H.; Yoshinari, M.; Kawada, E. Influence of sulfide concentration on the corrosion behavior of titanium in a simulated oral environment. Mater. Sci. Eng. C 2016, 62, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, M.; Jurczyk, M.A.U.; Miklaszewski, A.; Paszel-Jaworska, A.; Romaniuk, A.; Lipinska, N.; Zurawski, J.; Urbaniak, P.; Jurczyk, K. In vitro biocompatibility of titanium after plasma surface alloying with boron. Mater. Sci. Eng. C 2016, 69, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Revathi, A.; Borras, A.D.; Munoz, A.I.; Richard, C.; Manivasagam, G. Degradation mechanisms and future challenges of titanium and its alloys for dental implant applications in oral environment. Mater. Sci. Eng. C 2016, 76, 1354–1368. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N. Microbial ecosystem in the oral cavity: Metabolic diversity in an ecological niche and its relationship with oral diseases. Int. Congr. Ser. 2005, 1284, 103–112. [Google Scholar] [CrossRef]
- Parashar, A.; Parashar, S.; Zingade, A.; Gupta, S.; Sanikop, S. Interspecies communication in oral biofilm: An ocean of information. Oral Sci. Int. 2015, 12, 37–42. [Google Scholar] [CrossRef]
- Ferrer, M.D.; Mira, A. Oral biofilm architecture at the microbial scale. Trends Microbiol. 2016, 24, 246–248. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, Y.; Cao, Y.; Xue, J.; Zhou, X. The oral microbiome diversity and its relation to human diseases. Folia Microbiol. 2014, 60, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Bor, B.; Agnello, M.; Shi, W.; He, X. Ecology of the Oral Microbiome: Beyond Bacteria. Trends Microbiol. 2017, 25, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Microbiology of dental plaque biofilms and their role in oral health and caries. Dent. Clin. N. Am. 2010, 54, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Dental plaque: Biological significance of a biofilm and community life-style. J. Clin. Periodontol. 2005, 6, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival strategies of infectious biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Farah, C.S.; Ashman, R.B.; Challacombe, S.J. Oral candidosis. Front. Microbiol. 2000, 18, 553–562. [Google Scholar] [CrossRef]
- Maza, J.L.; Elguezabal, N.; Prado, C.; Ellacuría, J.; Soler, I.; Pontón, J. Candida albicans adherence to resin-composite restorative dental material: Influence of whole human saliva. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 94, 589–592. [Google Scholar] [CrossRef] [PubMed]
- Şen, B.H.; Safavi, K.E.; Spångberg, L.S.W. Colonization of Candida albicans on cleaned human dental hard tissues. Arch. Oral Biol. 1997, 42, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Rautemaa, R.; Ramage, G. Oral candidosis—Clinical challenges of a biofilm disease. Crit. Rev. Microbiol. 2011, 37, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Hannig, M.; Kensche, A.; Carpenter, G. The mucosal pellicle? An underestimated factor in oral physiology. Arch. Oral Biol. 2017, 80, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Glantz, P.O. Interfacial phenomena in the oral cavity. Colloids Surf. A Physicochem. Eng. Asp. 1997, 123–124, 657–670. [Google Scholar] [CrossRef]
- Bansil, R.; Turner, B.S. Mucin structure, aggregation, physiological functions and biomedical applications. Curr. Opin. Colloid Interface Sci. 2006, 11, 164–170. [Google Scholar] [CrossRef]
- Gibbins, H.L.; Yakubov, G.E.; Proctor, G.B.; Wilson, S.; Carpenter, G.H. What interactions drive the salivary mucosal pellicle formation? Colloids Surf. B Biointerfaces 2014, 120, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, R.; Osailan, S.M.; Challacombe, S.J.; Urquhart, D.; Proctor, G.B. Protein and mucin retention on oral mucosal surfaces in dry mouth patients. Eur. J. Oral Sci. 2010, 118, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Fouad, A.F.; Xu, H.H.K. Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dent. Mater. 2014, 30, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, C.J.; Klier, C.M.; Kolenbrander, P.E. Mechanisms of adhesion by oral bacteria. Annu. Rev. Microbiol. 1996, 50, 513–552. [Google Scholar] [CrossRef] [PubMed]
- Rosan, B.; Lamont, R.J. Dental plaque formation. Microbes Infect. 2000, 2, 1599–1607. [Google Scholar] [CrossRef]
- Li, Q.; Sand, W. Mechanical and chemical studies on EPS from Sulfobacillus thermosulfidooxidans: From planktonic to biofilm cells. Colloids Surf. B Biointerfaces 2017, 153, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.; Sissions, C.H. A comparison of human dental plaque microcosm biofilms grown in an undefined medium and a chemically defined artificial saliva. Arch. Oral Biol. 2001, 46, 477–486. [Google Scholar] [CrossRef]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.I.; Kaneko, K.; Moriyama, K.; Asaoka, K.; Sakai, J.I.; Nagumo, M. Hydrogen embrittlement of Ni-Ti superelastic alloy in fluoride solution. J. Biomed. Mater. Res. Part A 2003, 65, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.S.; Rodrigues, V.A.A.; Furtado, W.T.; Gueiros, S.; Pereira, G.S.; Avila-Campos, M.J. Microbial analysis of root canal and periradicular lesion associated to teeth with endodontic failure. Anaerobe 2017, 48, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, P.; Romero, M.; Vera, J.; Daood, U.; Khan, A.U.; Yan, A.; Cheung, G.S.P. Biofilms in endodontics current status and future directions. Int. J. Mol. Sci. 2017, 18, 21. [Google Scholar] [CrossRef] [PubMed]
- Mah, T.F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, T.H.; Tolker-Nielsen, T.; Givskov, M. Bacterial biofilm control by perturbation of bacterial signaling processes. Int. J. Mol. Sci. 2017, 18, 1970. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, K.K. What drives bacteria to produce a biofilm? FEMS Microbiol. Lett. 2004, 236, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Dental plaque as a microbial biofilm. Caries Res. 2004, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Slomiany, B.L.; Murty, V.L.N.; Piotrowski, J.; Slomiany, A. Salivary mucins in oral mucosal defense. Gen. Pharmacol. 1996, 27, 761–771. [Google Scholar] [CrossRef]
- Ranc, H.; Elkhyat, A.; Servais, C.; Mac-Mary, S.; Launay, B.; Humbert, P. Friction coefficient and wettability of oral mucosal tissue: Changes induced by a salivary layer. Colloids Surf. A Physicochem. Eng. Asp. 2006, 276, 155–161. [Google Scholar] [CrossRef]
- Vukosavljevic, D.; Custodio, W.; Buzalaf, M.A.R.; Hara, A.T.; Siqueira, W.L. Acquired pellicle as a modulator for dental erosion. Arch. Oral Biol. 2014, 59, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.D.; Meyer, J.; Taloumis, L.J. Intraoral galvanic corrosion—Literature-review and case-report. J. Prosthet. Dent. 1993, 69, 141–143. [Google Scholar] [CrossRef]
- Ciszewski, A.; Baraniaka, M.; Urbanek-Brychczynska, M. Corrosion by galvanic coupling between amalgam and different chromium-based alloys. Dent. Mater. 2007, 23, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Viennot, S.; Dalard, F.; Lissac, M.; Grosgogeat, B. Corrosion resistance of cobalt-chromium and palladium-silver alloys used in fixed prosthetic restorations. Eur. J. Oral Sci. 2005, 113, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, B.L.; Pederson, E.D.; Shklair, I.L. Salivary pH-rise activities in caries-free and caries-active naval recruits. Arch. Oral Biol. 1983, 28, 605–608. [Google Scholar] [CrossRef]
- Sutow, E.J.; Maillet, W.A.; Taylor, J.C.; Hall, G.C. In vivo galvanic currents of intermittently contacting dental amalgam and other metallic restorations. Dent. Mater. 2004, 20, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Hampf, G.; Ekholm, A.; Salo, T.; Ylipaavalniemi, P.; Aalberg, V.; Tuominen, S.; Alfthan, G. Pain in oral galvanism. Pain 1987, 29, 301–311. [Google Scholar] [CrossRef]
- Grushka, M.; Sessle, B.J.; Miller, R. Pain and personality profiles in burning mouth syndrome. Pain 1987, 28, 155–167. [Google Scholar] [CrossRef]
- Chang, J.-C.; Oshida, Y.; Gregory, R.L.; Andres, C.J.; Barco, T.M.; Brown, D.T. Electrochemical study on microbiology-related corrosion of metallic dental materials. Bio-Med. Mater. Eng. 2003, 13, 281–295. [Google Scholar]
- Beech, I.B.; Sunner, J. Biocorrosion: Towards understanding interactions between biofilms and metals. Curr. Opin. Biotechnol. 2004, 15, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Oda, H.; Ohkuma, K.; Sano, N.; Batbayar, N.; Terashima, Y.; Sato, S.; Terada, K. Microbiologically influenced corrosion of orthodontic metallic appliances. Dent. Mater. J. 2014, 33, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, T.P. An overview of the corrosion aspect of dental implants (titanium and its alloys). Indian J. Dent. Res. 2009, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Sanderson, B.J.S.; Wang, H. Cyto- and genotoxicity of ultrafine TiO2 particles in cultured human lymphoblastoid cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007, 628, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G.; Garhammer, P. Biological interactions of dental cast alloys with oral tissues. Dent. Mater. 2002, 18, 396–406. [Google Scholar] [CrossRef]
- Wataha, J.C. Biocompatibility of dental casting alloys: A review. J. Prosthet. Dent. 2000, 83, 223–234. [Google Scholar] [CrossRef]
- Jang, Y.; Owuor, D.; Waterman, J.T.; White, L.; Collins, B.; Sankar, J.; Gilbert, T.W.; Yun, Y. Effect of mucin and bicarbonate ion on corrosion behavior of AZ31 magnesium alloy for airway stents. Materials 2014, 7, 5866–5882. [Google Scholar] [CrossRef] [PubMed]
- Valero Vidal, C.; Igual Muñoz, A. Bio-Tribocorrosion in Biomaterials and Medical Implants; Woodhead Publishing: Sawston, UK, 2013; pp. 187–219. [Google Scholar]
- Khan, M.A.; Williams, R.L.; Williams, D.F. The corrosion behavior of Ti-6Al-4V, Ti-6Al-7Nb and Ti-13Nb-13Zr in protein solutions. Biomaterials 1999, 20, 631–637. [Google Scholar] [CrossRef]
- Busscher, H.J.; Rinastiti, M.; Siswomihardjo, W.; van der Mei, H.C. Biofilm formation on dental restorative and implant materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Shinde, R.B.; Raut, J.S.; Karuppayil, M.S. Biofilm formation by Candida albicans on various prosthetic materials and its fluconazole sensitivity: A kinetic study. Mycoscience 2012, 53, 220–226. [Google Scholar] [CrossRef]
- Williams, D.; Lewis, M. Pathogenesis and treatment of oral candidosis. J. Oral Microbiol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.J.; Savage, N.W. Oral candidosis and the therapeutic use of antifungal agents in dentistry. Aust. Dent. J. 2005, 50, 36–39. [Google Scholar] [CrossRef]
- Jones, D.A.; Amy, P.S. A thermodynamic interpretation of microbiologically influenced corrosion. Corrosion 2002, 58, 638–645. [Google Scholar] [CrossRef]
- Li, J.; Hirota, K.; Goto, T.; Yumoto, H.; Miyake, Y.; Ichikawa, T. Biofilm formation of Candida albicans on implant overdenture materials and its removal. J. Dent. 2012, 40, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.C.; Chan, K.Y.; Fang, H.H.P. Application of atomic force microscopy in the study of microbiologically influenced corrosion. Mater. Charact. 2002, 48, 195–203. [Google Scholar] [CrossRef]
- Hansen, D.C. Metal corrosion in the human body: The ultimate bio-corrosion scenario. Electrochem. Soc. Interface 2008, 17, 31–34. [Google Scholar] [CrossRef]
- Bahije, L.; Benyahia, H.; El Hamzaoui, S.; Ebn Touhami, M.; Bengueddour, R.; Rerhrhaye, W.; Abdallaoui, F.; Zaoui, F. Behavior of NiTi in the presence of oral bacteria: Corrosion by Streptococcus mutans. Int. Orthod. 2011, 9, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Beech, I.B.; Sunner, J.A.; Hiraoka, K. Microbe-surface interactions in biofouling and biocorrosion processes. Int. Microbiol. 2005, 157–168. [Google Scholar] [PubMed]
- Papadopoulou, K.; Eliades, T. Microbiologically-influenced corrosion of orthodontic alloys: A review of proposed mechanisms and effects. Aust. Orthod. J. 2009, 25, 63–75. [Google Scholar] [PubMed]
- Souza, J.C.M.; Ponthiaux, P.; Henriques, M.; Oliveira, R.; Teughels, W.; Celis, J.P.; Rocha, L.A. Corrosion behaviour of titanium in the presence of Streptococcus mutans. J. Dent. 2013, 41, 528–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, M.; Patel, H.; Kpendema, H.; Noar, J.H.; Hunt, N.P.; Mordan, N.J. Corrosion of intra-oral magnets by multi-species biofilms in the presence and absence of sucrose. Biomaterials 1997, 18, 53–57. [Google Scholar] [CrossRef]
- Lee, A.K.; Newman, D.K. Microbial iron respiration: Impacts on corrosion processes. Appl. Microbiol. Biotechnol. 2003, 62, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Zarasvand, K.A.; Rai, V.R. Microorganisms: Induction and inhibition of corrosion in metals. Int. Biodeterior. Biodegrad. 2014, 87, 66–74. [Google Scholar] [CrossRef]
- Dong, Z.H.; Liu, T.; Liu, H.F. Influence of EPS isolated from thermophilic sulphate-reducing bacteria on carbon steel corrosion. Biofouling 2011, 27, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Lata, S.; Sharma, C.; Singh, A.K. Comparison of biocorrosion due to Desulfovibrio desulfuricans and Desulfotomaculum nigrificans bacteria. J. Mater. Eng. Perform. 2013, 22, 463–469. [Google Scholar] [CrossRef]
- Loubinoux, J.; Mory, F.; Pereira, I.A.C.; Le Faou, A.E. Bacteremia caused by a strain of Desulfovibrio related to the provisionally named Desulfovibrio fairfieldensis. J. Clin. Microbiol. 2000, 38, 931–934. [Google Scholar] [PubMed]
- Nazina, T.N.; Rozanova, E.P.; Belyakova, E.V.; Lysenko, A.M.; Poltaraus, A.B.; Tourova, T.P.; Osipov, G.A.; Belyaev, S.S. Description of Desulfotomaculum nigrificans subsp. salinus as a new species, Desulfotomaculum salinum sp. nov. Microbiology 2005, 74, 567–574. [Google Scholar] [CrossRef]
- Lopes, F.A.; Morin, P.; Oliveira, R.; Melo, L.F. Interaction of Desulfovibrio desulfuricans biofilms with stainless steel surface and its impact on bacterial metabolism. J. Appl. Microbiol. 2006, 101, 1087–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muyzer, G.; Stams, A.J. The ecology and biotechnology of sulphate-reducing bacteria. Nat. Rev. Microbiol. 2008, 6, 441–454. [Google Scholar] [CrossRef] [PubMed]
- Mystkowska, J.; Ferreira, J.A.; Leszczyńska, K.; Chmielewska, S.; Dąbrowski, J.R.; Wieciński, P.; Kurzydłowski, K.J. Biocorrosion of 316LV steel used in oral cavity due to Desulfotomaculum nigrificans bacteria. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Mystkowska, J. Biocorrosion of dental alloys due to Desulfotomaculum nigrificans bacteria. Acta Bioeng. Biomech. 2016, 18, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Zuo, R. Biofilms: Strategies for metal corrosion inhibition employing microorganisms. Appl. Microbiol. Biotechnol. 2007, 76, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Q.; Song, Y.; Zhang, D.; Yu, S.; Zhu, X. A study on the corrosion behavior of co-modified cast AZ91 magnesium alloy in the presence of sulfate-reducing bacteria. J. Alloys Compd. 2009, 473, 550–556. [Google Scholar] [CrossRef]
- Lata, S.; Sharma, C.; Singh, A.K. Effect of host media on microbial influenced corrosion due to Desulfotomaculum nigrificans. J. Mater. Eng. Perform. 2013, 22, 1120–1128. [Google Scholar] [CrossRef]
- Lata, S.; Sharma, C.; Singh, A.K. Microbial influenced corrosion by thermophilic bacteria. J. Corros. Sci. Eng. 2011, 14. [Google Scholar] [CrossRef]
- Songür, M.; Çelikkan, H.; Gökmeşe, F.; Şimşek, S.A.; Altun, N.Ş.; Aksu, M.L. Electrochemical corrosion properties of metal alloys used in orthopedic implants. J. Appl. Electrochem. 2009, 39. [Google Scholar] [CrossRef]
- Gurappa, I. Characterization of different materials for corrosion resistance under simulated body fluid conditions. Mater. Charact. 2002, 49, 73–79. [Google Scholar] [CrossRef]
- Hodgson, A.W.E.; Kurz, S.; Virtanen, S.; Fervel, V.; Olsson, C.O.A.; Mischler, S. Passive and transpassive behaviour of CoCrMo in simulated biological solutions. Electrochim. Acta 2004, 49, 2167–2178. [Google Scholar] [CrossRef]
- Baskaran, V.; Nemati, M. Anaerobic reduction of sulfate in immobilized cell bioreactors, using a microbial culture originated from an oil reservoir. Biochem. Eng. J. 2006, 31, 148–159. [Google Scholar] [CrossRef]
- Geesey, G.G.; Gillis, R.J.; Avci, R.; Daly, D.; Hamilton, M.; Shope, P.; Harkin, G. The influence of surface features on bacterial colonization and subsequent substratum chemical changes of 316l stainless steel. Corros. Sci. 1996, 38, 73–95. [Google Scholar] [CrossRef]
- Chen, G.; Clayton, C.R. Influence of sulfate-reducing bacteria on the passivity of type 304 austenitic stainless steel. J. Electrochem. Soc. 1997, 144, 3140–3146. [Google Scholar] [CrossRef]
- Kumar, A.V.R.; Singh, R.; Nigam, R.K. Mossbauer spectroscopy of corrosion products of mild steel due to microbiologically influenced corrosion. J. Radioanal. Nucl. Chem. 1999, 242, 131–137. [Google Scholar] [CrossRef]
- Campbell, A.G.; Campbell, J.H.; Schwientek, P.; Woyke, T.; Sczyrba, A.; Allman, S.; Beall, C.J.; Griffen, A.; Leys, E.; Podar, M. Multiple single-cell genomes provide insight into functions of uncultured deltaproteobacteria in the human oral cavity. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Chatelus, C.; Carrier, P.; Saignes, P.; Libert, M.F.; Berlier, Y.; Lespinat, P.A.; Fauque, G.; Legall, J. Hydrogenase activity in aged, nonviable Desulfovibrio vulgaris cultures and its significance in anaerobic biocorrosion. Appl. Environ. Microbiol. 1987, 53, 1708–1710. [Google Scholar] [PubMed]
- Bryant, R.D.; Laishley, E.J. The role of hydrogenase in anaerobic biocorrosion. Can. J. Microbiol. 1990, 36, 259–264. [Google Scholar] [CrossRef]
- Gu, J.D.; Ford, T.E.; Mitchell, R. Microbiological Corrosion of Metallic Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 549–557. [Google Scholar]
- Örnek, D.; Wood, T.K.; Hsu, C.H.; Sun, Z.; Mansfeld, F. Pitting corrosion control of aluminum 2024 using protective biofilms that secrete corrosion inhibitors. Corrosion 2002, 58, 761–767. [Google Scholar] [CrossRef]
- Jayaraman, A.; Mansfeld, F.B.; Wood, T.K. Inhibiting sulfate-reducing bacteria in biofilms by expressing the antimicrobial peptides indolicidin and bactenecin. J. Ind. Microbiol. Biotechnol. 1999, 22, 167–175. [Google Scholar] [CrossRef]
- D'Incau, E.; Couture, C.; Maureille, B. Human tooth wear in the past and the present: Tribological mechanisms, scoring systems, dental and skeletal compensations. Arch. Oral Biol. 2012, 57, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, Z.R. Friction and wear behavior of human teeth under various wear conditions. Tribol. Int. 2007, 40, 278–284. [Google Scholar] [CrossRef]
- Dabrowski, J.R.; Klekotka, M.; Sidun, J. Fretting and fretting corrosion of 316L implantation steel in oral cavity environment. Eksploatacja i Niezawodność Maint. Reliab. 2014, 16, 441–446. [Google Scholar]
- Andrysewicz, E.; Mystkowska, J.; Dabrowski, J.R.; Olchowik, R. Influence of self-made saliva substitutes on tribological characteristics of human enamel. Acta Bioeng. Biomech. 2014, 16, 67–74. [Google Scholar] [PubMed]
- Reeh, E.S.; Douglas, W.H.; Levine, M.J. Lubrication of saliva substitutes at enamel-to-enamel contacts in an artificial mouth. J. Prosthet. Dent. 1996, 75, 649–656. [Google Scholar] [CrossRef]
- Ranc, H.; Servais, C.; Chauvy, P.F.; Debaud, S.; Mischler, S. Effect of surface structure on frictional behaviour of a tongue/palate tribological system. Tribol. Int. 2006, 39, 1518–1526. [Google Scholar] [CrossRef]
- Andrysewicz, E.; Mystkowska, J.; Kolmas, J.; Jalbrzykowski, M.; Olchowik, R.; Dabrowski, J.R. Influence of artificial saliva compositions on tribological characteristics of Ti-6Al-4V implant alloy. Acta Bioeng. Biomech. 2012, 14, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Douglas, W.H.; Reeh, E.S.; Ramasubbu, N.; Raj, P.A.; Bhandary, K.K.; Levine, M.J. Statherin: A major boundary lubricant of human saliva. Biochem. Biophys. Res. Commun. 1991, 180, 91–97. [Google Scholar] [CrossRef]
- Yakubov, G.E.; McColl, J.; Bongaerts, J.H.H.; Ramsden, J.J. Viscous boundary lubrication of hydrophobic surfaces by mucin. Langmuir 2009, 25, 2313–2321. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, M.; Arnebrandt, T.; Rennie, A.; Fragneto, G.; Thomas, R.K.; Lindh, L. Human saliva forms a complex film structure on alumina surfaces. Biomacromolecules 2007, 8, 65–69. [Google Scholar] [CrossRef] [PubMed]
- MacAkova, L.; Yakubov, G.E.; Plunkett, M.A.; Stokes, J.R. Influence of ionic strength on the tribological properties of pre-adsorbed salivary films. Tribol. Int. 2011, 44, 956–962. [Google Scholar] [CrossRef]
- Yakubov, G.E.; Macakova, L.; Wilson, S.; Windust, J.H.C.; Stokes, J.R. Aqueous lubrication by fractionated salivary proteins: Synergistic interaction of mucin polymer brush with low molecular weight macromolecules. Tribol. Int. 2015, 89, 34–45. [Google Scholar] [CrossRef]
- Harvey, N.M.; Yakubov, G.E.; Stokes, J.R.; Klein, J. Lubrication and load-bearing properties of human salivary pellicles adsorbed ex vivo on molecularly smooth substrata. Biofouling 2012, 28, 843–856. [Google Scholar] [CrossRef] [PubMed]
- Towler, B.W.; Rupp, C.J.; Cunningham, A.B.; Stoodley, P. Viscoelastic properties of a mixed culture biofilm from rheometer creep analysis. Biofouling 2003, 19, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Hazra, S.; Choudhury, M.D.; Sinha, S.D.; Das, S.; Middya, T.R.; Tarafdar, S.; Dutta, T. A study of the rheological properties of visco-elastic materials using fractional calculus. Colloids Surf. A Physicochem. Eng. Asp. 2017, 516, 181–189. [Google Scholar] [CrossRef]
- Sutherland, I.W. The biofilm matrix—An immobilized but dynamic microbial environment. Trends Microbiol. 2001, 9, 222–227. [Google Scholar] [CrossRef]
- Mathew, M.T.; Barão, V.A.; Yuan, J.C.C.; Assunção, W.G.; Sukotjo, C.; Wimmer, M.A. What is the role of lipopolysaccharide on the tribocorrosive behavior of titanium? J. Mech. Behav. Biomed. Mater. 2012, 8, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Messer, R.L.W.; Tackas, G.; Mickalonis, J.; Brown, Y.; Lewis, J.B.; Wataha, J.C. Corrosion of machined titanium dental implants under inflammatory conditions. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-García, A.; Pérez-Alvarez, J.; Barrera, C.C.; Medina, J.C.; Almaguer-Flores, A.; Sánchez, R.B.; Rodil, S.E. The effect of simulated inflammatory conditions on the surface properties of titanium and stainless steel and their importance as biomaterials. Mater. Sci. Eng. C 2016, 66, 119–129. [Google Scholar] [CrossRef] [PubMed]







| Oral Bacteria Microbiome | |
| Saliva | Actinobacteria, Bacteroides, Firmicutes, Fusobacteria, Proteobacteria, Spirochaetes, TM7 (The Human Microbiome Consortium) |
| Dental plaque | Firmicutes, Actinobacteria |
| Oral mucosa | Streptococcus salivarius, Rothia mucilaginosa, Eubacterium strain FTB41 |
| Oral Bacteria Related to Oral Diseases | |
| Dental caries | Streptococcus, Veillonella, Actinomyces, Granulicatella, Leptotrichia, Thiomonas, Bifidobacterium, Prevotella, Lactobacillus, Propionibacterium, Pseudoramibacter, Selenomonas |
| Periapical infections (Periapical periodontitis, root canal infection) | Proteobacteria, Firmicutes, Bacteroidetes, Fusobacteria, Actinobacteria, Olsenella uli, Prevotella baroniae, Porphyromonas endodontalis, Fusobacterium nucleatum, Tannerella forsythia, Propionibacterium propionicum, Porphyromonas gingivalis, Prevotella intermedia, Prevotella oralis, Parvimonas micra, Porphyromonas endodontalis, Fusobacterium nucleatum, Tannerella forsythia |
| Periodontal diseases (Gingivitis, Periodontitis) | Actinomycetes, Capnocytophaga, Campylobacter, Eikenella, Fusobacterium, Prevotella, Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia, Bacteroidetes spp., Eubacterium saphenum, Porphyromonas endodontalis, Prevotella denticola, Parvimonas micra, Peptostreptococcus spp., Filifactor alocis, Desulfobulbus spp., Dialister spp., Synergistetes |
| Halitosis | Solobacterium moorei, Atopobium parvulum, Eubacterium sulci |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mystkowska, J.; Niemirowicz-Laskowska, K.; Łysik, D.; Tokajuk, G.; Dąbrowski, J.R.; Bucki, R. The Role of Oral Cavity Biofilm on Metallic Biomaterial Surface Destruction–Corrosion and Friction Aspects. Int. J. Mol. Sci. 2018, 19, 743. https://doi.org/10.3390/ijms19030743
Mystkowska J, Niemirowicz-Laskowska K, Łysik D, Tokajuk G, Dąbrowski JR, Bucki R. The Role of Oral Cavity Biofilm on Metallic Biomaterial Surface Destruction–Corrosion and Friction Aspects. International Journal of Molecular Sciences. 2018; 19(3):743. https://doi.org/10.3390/ijms19030743
Chicago/Turabian StyleMystkowska, Joanna, Katarzyna Niemirowicz-Laskowska, Dawid Łysik, Grażyna Tokajuk, Jan R. Dąbrowski, and Robert Bucki. 2018. "The Role of Oral Cavity Biofilm on Metallic Biomaterial Surface Destruction–Corrosion and Friction Aspects" International Journal of Molecular Sciences 19, no. 3: 743. https://doi.org/10.3390/ijms19030743
APA StyleMystkowska, J., Niemirowicz-Laskowska, K., Łysik, D., Tokajuk, G., Dąbrowski, J. R., & Bucki, R. (2018). The Role of Oral Cavity Biofilm on Metallic Biomaterial Surface Destruction–Corrosion and Friction Aspects. International Journal of Molecular Sciences, 19(3), 743. https://doi.org/10.3390/ijms19030743