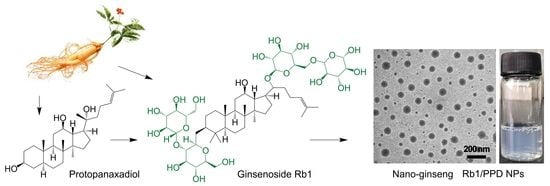
“Nano-Ginseng” for Enhanced Cytotoxicity AGAINST Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. Formulation of Rb1/PPD Nanoparticles (NPs)
2.2. Drug Stability In Vitro
2.3. Hemolysis Study
2.4. In Vitro Cytotoxicity
2.5. Glucose Competition
2.6. In Vivo Biodistribution and Pharmacokinetics
2.7. In Vivo Anticancer Activities
3. Materials and Methods
3.1. Materials
3.2. Animals and Ethics
3.3. Preparation of Rb1/PPD Nanoparticles (NPs)
3.4. Characterization of Nanoparticles
3.5. Drug Loading and Release
3.6. Hemolysis Assay
3.7. In Vitro Cell Cytotoxicity
3.8. Pharmacokinetic Experiments
3.9. In Vivo Biodistribution
3.10. In Vivo Efficacy Studies
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nag, S.A.; Qin, J.-J.; Wang, W.; Wang, M.-H.; Wang, H.; Zhang, R. Ginsenosides as anticancer agents: In vitro and in vivo activities, structure–activity relationships, and molecular mechanisms of action. Front. Pharmacol. 2012, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.-W.; Wang, C.-Z.; Yuan, C.-S. American ginseng: Potential structure–function relationship in cancer chemoprevention. Biochem. Pharmacol. 2010, 80, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P. Chapter 1 ginsenosides: Chemistry, biosynthesis, analysis, and potential health effects. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2008; Volume 55, pp. 1–99. [Google Scholar]
- Zhu, G.-Y.; Li, Y.-W.; Tse, A.K.-W.; Hau, D.K.-P.; Leung, C.-H.; Yu, Z.-L.; Fong, W.-F. 20(S)-protopanaxadiol, a metabolite of ginsenosides, induced cell apoptosis through endoplasmic reticulum stress in human hepatocarcinoma HepG2 cells. Eur. J. Pharmacol. 2011, 668, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Z.; Wu, X.; Zhang, C.-F.; Calway, T.; He, T.-C.; Du, W.; Chen, J.; Wang, C.-Z.; Yuan, C.-S. Trail pathway is associated with inhibition of colon cancer by protopanaxadiol. J. Pharmacol. Sci. 2015, 127, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, X.; Li, D.; Zhong, D. Identification of 20(S)-protopanaxadiol metabolites in human liver microsomes and human hepatocytes. Drug Metab. Dispos. 2011, 39, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, H.; Bao, G. Physical principles of nanoparticle cellular endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Hennink, W.E.; Storm, G. Tumour-targeted nanomedicines: Principles and practice. Br. J. Cancer 2008, 99, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and nanomedicine for nanoparticle-based diagnostics and therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Rösler, A.; Vandermeulen, G.W.M.; Klok, H.-A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev. 2012, 64, 270–279. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Jeong, H.G. Ginsenoside rb1 protects against 6-hydroxydopamine-induced oxidative stress by increasing heme oxygenase-1 expression through an estrogen receptor-related PI3K/Akt/Nrf2-dependent pathway in human dopaminergic cells. Toxicol. Appl. Pharmacol. 2010, 242, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.W.; Cheung, L.W.T.; Pon, Y.L.; Wong, R.N.S.; Mak, N.K.; Fan, T.P.P.; Au, S.C.L.; Tombran Tink, J.; Wong, A.S.T. Ginsenoside Rb1 inhibits tube-like structure formation of endothelial cells by regulating pigment epithelium-derived factor through the oestrogen β receptor. Br. J. Pharmacol. 2007, 152, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Toward a full understanding of the epr effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Zhang, X.; Lu, J.; Huang, Y.; Zhao, W.; Chen, Y.; Li, J.; Gao, X.; Venkataramanan, R.; Sun, M.; Stolz, D.B.; et al. Peg-farnesylthiosalicylate conjugate as a nanomicellar carrier for delivery of paclitaxel. Bioconjug. Chem. 2013, 24, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.J.; Lmd, G. Chitosan nanoparticles as a mucoadhesive drug delivery system for ocular administration. Mar. Drugs 2017, 15, 370. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Liu, P.; Deng, R.; Lei, M.; Chen, W.; Hu, L. 20(S)-protopanaxadiol (PPD) analogues chemosensitize multidrug-resistant cancer cells to clinical anticancer drugs. Bioorg. Med. Chem. 2013, 21, 4279–4287. [Google Scholar] [CrossRef] [PubMed]
- El Mjiyad, N.; Caro-Maldonado, A.; Ramírez-Peinado, S.; Muñoz-Pinedo, C. Sugar-free approaches to cancer cell killing. Oncogene 2010, 30, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Unger, F.; Wittmar, M.; Kissel, T. Branched polyesters based on poly[vinyl-3-(dialkylamino)alkylcarbamate-co-vinyl acetate-co-vinyl alcohol]-graft-poly(d,l-lactide-co-glycolide): Effects of polymer structure on cytotoxicity. Biomaterials 2007, 28, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Fang, C.; Pei, Y. Stealth peg-phdca niosomes: Effects of chain length of peg and particle size on niosomes surface properties, in vitro drug release, phagocytic uptake, in vivo pharmacokinetics and antitumor activity. J. Pharm. Sci. 2006, 95, 1873–1887. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Yu, X.N.; Kubo, C. Dehydroepiandrosterone attenuates the spontaneous elevation of serum ige level in NC/Nga mice. Immunol. Lett. 2001, 79, 177–179. [Google Scholar] [CrossRef]
- Matsuda, H.; Watanabe, N.; Geba, G.P.; Sperl, J.; Tsudzuki, M.; Hiroi, J.; Matsumoto, M.; Ushio, H.; Saito, S.; Askenase, P.W. Development of atopic dermatitis-like skin lesion with ige hyperproduction in NC/Nga mice. Int. Immunol. 1997, 9, 461–466. [Google Scholar] [CrossRef] [PubMed]





| Compound | Size (nm) | PDI | ζ-Potential (mV) | DLE (%) | DLC (wt %) | Yield (%) |
|---|---|---|---|---|---|---|
| Rb1 NPs | 78.9 ± 8.2 | 0.061 ± 0.002 | −15.8 ± 0.9 | - | - | |
| Rb1/PPD NPs | 122.5 ± 10.8 | 0.089 ± 0.005 | −13.2 ± 0.6 | 96.8 ± 1.2 | 27.9 ± 1.4 | 89.6 ± 1.8 |
| pH | First Order | Hixson–Crowell | Higuchi | Korsmeyer–Peppas | |||||
|---|---|---|---|---|---|---|---|---|---|
| k | r2 | k | r2 | k | r2 | k | n | r2 | |
| 7.4 | 0.038 | 0.744 | 0.064 | 0.675 | 10.476 | 0.991 | 0.100 | 0.527 | 0.976 |
| 6.8 | 0.029 | 0.715 | 0.065 | 0.618 | 11.246 | 0.957 | 0.188 | 0.394 | 0.949 |
| Compound | IC50 (μg/mL) | Mean TV ± SD (mm3) 1 | RTV 1 | TGI (%) 1 | Cures (%) 1 |
|---|---|---|---|---|---|
| normal saline | - | 4225 ± 1748 | 37.0 ± 15.2 | 0 | 0 |
| Rb1 NPs | - | 4273 ± 1142 | 41.9 ± 11.2 | - | 0 |
| PPD | 78.81 ± 5.36 | 3526 ± 1220 | 31.2 ± 10.8 | 16.6 | 33.3 |
| Rb1 + PPD | 69.16 ± 5.08 | 3338 ± 1086 | 29.8 ± 9.7 | 21.0 | 50.0 |
| Rb1/PPD NPs | 35.32 ± 4.30 | 1322 ± 507 | 11.2 ± 4.3 | 68.7 | 66.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, L.; Zhu, W.; Si, C.; Lei, J. “Nano-Ginseng” for Enhanced Cytotoxicity AGAINST Cancer Cells. Int. J. Mol. Sci. 2018, 19, 627. https://doi.org/10.3390/ijms19020627
Dai L, Zhu W, Si C, Lei J. “Nano-Ginseng” for Enhanced Cytotoxicity AGAINST Cancer Cells. International Journal of Molecular Sciences. 2018; 19(2):627. https://doi.org/10.3390/ijms19020627
Chicago/Turabian StyleDai, Lin, Weiyan Zhu, Chuanling Si, and Jiandu Lei. 2018. "“Nano-Ginseng” for Enhanced Cytotoxicity AGAINST Cancer Cells" International Journal of Molecular Sciences 19, no. 2: 627. https://doi.org/10.3390/ijms19020627
APA StyleDai, L., Zhu, W., Si, C., & Lei, J. (2018). “Nano-Ginseng” for Enhanced Cytotoxicity AGAINST Cancer Cells. International Journal of Molecular Sciences, 19(2), 627. https://doi.org/10.3390/ijms19020627