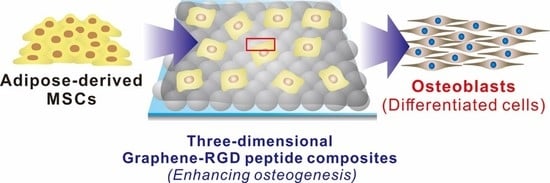
Three-Dimensional Graphene–RGD Peptide Nanoisland Composites That Enhance the Osteogenesis of Human Adipose-Derived Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Results
2.1. Fabrication of Silica-Nanoparticle–Graphene-Oxide Composites on an Indium Tin Oxide (ITO) Substrate
2.2. Fabrication of a 3D Graphene–RGD Peptide Nanoisland Composite for Stem Cell Cultures
2.3. Guiding ADSC Osteogenesis Using Graphene–RGD Peptide Nanoisland Composites
2.4. Time Course of ADSC Osteogenic Differentiation on Graphene–RGD Peptide Nanoislands
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Fabrication of Three-Dimensional Hybrid Structures
4.3. Substrate Characterization
4.4. Cell Proliferation and Spreading Analysis
4.5. Osteogenesis of ADSCs
4.6. Osteogenic Marker Expression Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ADSC | Adipose-derived mesenchymal stem cells |
| ALP | Alkaline phosphatase |
| ARS | Alizarin red S |
| DW | Distilled water |
| ECM | Extracellular matrix |
| ITO | Indium tin oxide |
| GNP | Gold nanoparticle |
| GO | Graphene oxide |
| PDMS | Polydimethylsilozane |
| RGD | Arginine-glycine-aspartic acid |
| RT-qPCR | Reverse transcription quantitative polymerase chain reaction |
| RUNX2 | Runt-related transcription factor 2 |
| SEM | Scanning electron microscopy |
| SiNPs | Silica nanoparticles |
| XPS | X-ray photoelectron spectroscopy |
References
- Segers, V.F.; Lee, R.T. Stem-cell therapy for cardiac disease. Nature 2008, 451, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Strauer, B.E.; Kornowski, R. Stem cell therapy in perspective. Circulation 2003, 107, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Ankrum, J.; Karp, J.M. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol. Med. 2010, 16, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Hui, J.H.; Song, I.C.; Ardany, L.; Lee, E.H. Injectable mesenchymal stem cell therapy for large cartilage defects—A porcine model. Stem Cells 2007, 25, 2964–2971. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Campagnoli, C.; Roberts, I.A.; Kumar, S.; Bennett, P.R.; Bellantuono, I.; Fisk, N.M. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 2001, 98, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Curran, J.M.; Chen, R.; Hunt, J.A. The guidance of human mesenchymal stem cell differentiation in vitro by controlled modifications to the cell substrate. Biomaterials 2006, 27, 4783–4793. [Google Scholar] [CrossRef] [PubMed]
- Suhito, I.R.; Han, Y.; Min, J.; Son, H.; Kim, T.-H. In situ label-free monitoring of human adipose-derived mesenchymal stem cell differentiation into multiple lineages. Biomaterials 2018, 154, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, M.K.; Keane-Moore, M.; Buyaner, D.; Hardy, W.B.; Moorman, M.A.; McIntosh, K.R.; Mosca, J.D. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J. Biomed. Sci. 2003, 10, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Bruder, S.P. Mesenchymal stem cells: Building blocks for molecular medicine in the 21st century. Trends Mol. Med. 2001, 7, 259–264. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Mackay, A.M.; Beck, S.C.; Murphy, J.M.; Barry, F.P.; Chichester, C.O.; Pittenger, M.F. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998, 4, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Kim, Y.J.; Son, Y.J.; Yoo, H.S.; Park, J.H. Promotive effects of human induced pluripotent stem cell-conditioned medium on the proliferation and migration of dermal fibroblasts. Biotechnol. Bioproc. Eng. 2017, 22, 561–568. [Google Scholar] [CrossRef]
- Shih, Y.R.V.; Tseng, K.F.; Lai, H.Y.; Lin, C.H.; Lee, O.K. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J. Bone Miner Res. 2011, 26, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef] [PubMed]
- Knippenberg, M.; Helder, M.N.; Zandieh, D.B.; Semeins, C.M.; Wuisman, P.I.; Klein-Nulend, J. Adipose tissue-derived mesenchymal stem cells acquire bone cell-like responsiveness to fluid shear stress on osteogenic stimulation. Tissue Eng. 2005, 11, 1780–1788. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.M.; Park, J.-S.; Jeong, S.I.; An, S.-J.; Gwon, H.-J.; Lim, Y.-M.; Nho, Y.-C.; Kim, C.-Y. Promotion of human mesenchymal stem cell differentiation on bioresorbable polycaprolactone/biphasic calcium phosphate composite scaffolds for bone tissue engineering. Biotechnol. Bioproc. Eng. 2014, 19, 341–349. [Google Scholar] [CrossRef]
- Nava, M.M.; Raimondi, M.T.; Pietrabissa, R. Controlling self-renewal and differentiation of stem cells via mechanical cues. Biom. Res. Int. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Connelly, J.T.; Gautrot, J.E.; Trappmann, B.; Tan, D.W.-M.; Donati, G.; Huck, W.T.; Watt, F.M. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat. Cell Biol. 2010, 12, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.-W.; Lin, H.-I.; Ho, J.H.-C.; Shih, Y.-R.V.; Chen, H.-F.; Yen, T.-J.; Lee, O.K. Regulation of the fate of human mesenchymal stem cells by mechanical and stereo-topographical cues provided by silicon nanowires. Biomaterials 2012, 33, 5013–5022. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Gilbert, P.M.; Blau, H.M. Designing materials to direct stem-cell fate. Nature 2009, 462, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, C.S.; Fu, J. Forcing stem cells to behave: A biophysical perspective of the cellular microenvironment. Ann. Rev. Biophys. 2012, 41, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Li, J.Y.S.; Yeh, Y.; Yang, L.; Chien, S. Effects of matrix elasticity and cell density on human mesenchymal stem cells differentiation. J. Orthop. Res. 2013, 31, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Suhito, I.R.; Han, Y.; Kim, D.-S.; Son, H.; Kim, T.-H. Effects of two-dimensional materials on human mesenchymal stem cell behaviors. Biochem. Biophys. Res. Commun. 2017, 493, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Ragetly, G.R.; Griffon, D.J.; Lee, H.-B.; Fredericks, L.P.; Gordon-Evans, W.; Chung, Y.S. Effect of chitosan scaffold microstructure on mesenchymal stem cell chondrogenesis. Acta Biomater. 2010, 6, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, P.; Helen, W.; Engler, A.J.; Levchenko, A.; Kim, D.-H. Control of stem cell fate and function by engineering physical microenvironments. Integr. Biol. 2012, 4, 1008–1018. [Google Scholar] [CrossRef]
- Huri, P.Y.; Cook, C.; Hutton, D.; Goh, B.; Gimble, J.; DiGirolamo, D.; Grayson, W.L. Biophysical cues enhance myogenesis of human adipose derived stem/stromal cells. Biochem. Biophys. Res. Commun. 2013, 438, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Dai, J.; Zhang, X.A. Environmental physical cues determine the lineage specification of mesenchymal stem cells. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Keung, A.J.; Healy, K.E.; Kumar, S.; Schaffer, D.V. Biophysics and dynamics of natural and engineered stem cell microenvironments. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Chueng, S.-T.D.; Yang, L.; Zhang, Y.; Lee, K.-B. Multidimensional nanomaterials for the control of stem cell fate. Nano. Converg. 2016, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.C.; Lim, C.H.Y.; Shi, H.; Tang, L.A.; Wang, Y.; Lim, C.T.; Loh, K.P. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 2011, 5, 7334–7341. [Google Scholar] [CrossRef] [PubMed]
- Nayak, T.R.; Andersen, H.; Makam, V.S.; Khaw, C.; Bae, S.; Xu, X.; Ee, P.-L.R.; Ahn, J.-H.; Hong, B.H.; Pastorin, G.; et al. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 2011, 5, 4670–4678. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Park, J.; Sim, S.H.; Sung, M.G.; Kim, K.S.; Hong, B.H.; Hong, S. Enhanced differentiation of human neural stem cells into neurons on graphene. Adv. Mater. 2011, 23. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.-S.; Kim, D.-S.; Suhito, I.R.; Choo, S.-S.; Kim, S.-J.; Song, I.; Kim, T.H. Guiding osteogenesis of mesenchymal stem cells using carbon-based nanomaterials. Nano Converg. 2017, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Lee, T.; El-Said, W.A.; Choi, J.-W. Graphene-based materials for stem cell applications. Materials 2015, 8, 8674–8690. [Google Scholar] [CrossRef] [PubMed]
- Janderová, L.; McNeil, M.; Murrell, A.N.; Mynatt, R.L.; Smith, S.R. Human mesenchymal stem cells as an in vitro model for human adipogenesis. Obesity 2003, 11, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Luu, Y.K.; Capilla, E.; Rosen, C.J.; Gilsanz, V.; Pessin, J.E.; Judex, S.; Rubin, C.T. Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J. Bone. Miner Res. 2009, 24, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Bruder, S.P.; Fink, D.J.; Caplan, A.I. Mesenchymal stem cells in bone development, bone repair, and skeletal regenaration therapy. J. Cell Biochem. 1994, 56, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Fraser, J.L.; Lu, Z.-Y.; Hu, X.; Yu, S.P. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol. Dis. 2012, 46, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Mooney, E.; Dockery, P.; Greiser, U.; Murphy, M.; Barron, V. Carbon nanotubes and mesenchymal stem cells: Biocompatibility, proliferation and differentiation. Nano Lett. 2008, 8, 2137–2143. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Namgung, S.; Kim, B.; Im, J.; Kim, J.; Sun, K.; Lee, K.B.; Nam, J.M.; Park, Y.; Hong, S. Carbon nanotube monolayer patterns for directed growth of mesenchymal stem cells. Adv. Mater. 2007, 19, 2530–2534. [Google Scholar] [CrossRef]
- Baik, K.Y.; Park, S.Y.; Heo, K.; Lee, K.B.; Hong, S. Carbon nanotube monolayer cues for osteogenesis of mesenchymal stem cells. Small 2011, 7, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E.; Emamy, H.; Akhavan, F. Genotoxicity of graphene nanoribbons in human mesenchymal stem cells. Carbon 2013, 54, 419–431. [Google Scholar] [CrossRef]
- Trappmann, B.; Gautrot, J.E.; Connelly, J.T.; Strange, D.G.; Li, Y.; Oyen, M.L.; Cohen Stuart, M.A.; Boehm, H.; Li, B.; Vogel, V.; et al. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012, 11, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Watt, F.M.; Huck, W.T. Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell Biol. 2013, 14, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, A.; Hennessy, K.; Bellis, S. Regulation of mesenchymal stem cell attachment and spreading on hydroxyapatite by RGD peptides and adsorbed serum proteins. Biomaterials 2005, 26, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Park, T.G. Biomimicking extracellular matrix: Cell adhesive RGD peptide modified electrospun poly (d, l-lactic-co-glycolic acid) nanofiber mesh. Tissue Eng. 2006, 12, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Lee, D.; Choi, J.-W. Live cell biosensing platforms using graphene-based hybrid nanomaterials. Biosens. Bioelectron. 2017, 94, 485–499. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Peng, G.; Li, J.; Ma, P.; Wang, Z.; Shu, W.; Peng, S.; Chen, G.-Q. Chondrogenic differentiation of human bone marrow mesenchymal stem cells on polyhydroxyalkanoate (PHA) scaffolds coated with PHA granule binding protein PhaP fused with RGD peptide. Biomaterials 2011, 32, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, A.; Weeks, D.; Kelpke, S.; McCracken, M.S.; Bellis, S. The effect of the addition of a polyglutamate motif to RGD on peptide tethering to hydroxyapatite and the promotion of mesenchymal stem cell adhesion. Biomaterials 2005, 26, 7046–7056. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Tegenfeldt, J.O.; Austin, R.H.; Chou, S.Y. Gradient nanostructures for interfacing microfluidics and nanofluidics. Appl. Phys. Lett. 2002, 81, 3058–3060. [Google Scholar] [CrossRef]
- Lowe, C.R. Nanobiotechnology: The fabrication and applications of chemical and biological nanostructures. Curr. Opin. Struct. Biol. 2000, 10, 428–434. [Google Scholar] [CrossRef]
- Lu, C.-W.; Hung, Y.; Hsiao, J.-K.; Yao, M.; Chung, T.-H.; Lin, Y.-S.; Wu, S.H.; Hsu, S.C.; Liu, H.M.; Mou, C.Y.; et al. Bifunctional magnetic silica nanoparticles for highly efficient human stem cell labeling. Nano Lett. 2007, 7, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Bharali, D.J.; Klejbor, I.; Stachowiak, E.K.; Dutta, P.; Roy, I.; Kaur, N.; Bergey, E.J.; Prasad, P.N.; Stachowiak, M.K. Organically modified silica nanoparticles: A nonviral vector for in vivo gene delivery and expression in the brain. Proc. Natl. Acad. Sci. USA 2005, 102, 11539–11544. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 1992, 69, 11–25. [Google Scholar] [CrossRef]
- Grinnell, B.W.; Hermann, R.B.; Yan, S.B. Human protein C inhibits selectin-mediated cell adhesion: Role of unique fucosylated oligosaccharide. Glycobiology 1994, 4, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, M. Cadherins: A molecular family important in selective cell-cell adhesion. Annu. Rev. Biochem. 1990, 59, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Albelda, S. Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab. Investig. 1993, 68, 4–17. [Google Scholar] [PubMed]
- Chapman, H.A. Plasminogen activators, integrins, and the coordinated regulation of cell adhesion and migration. Curr. Opin. Cell Biol. 1997, 9, 714–724. [Google Scholar] [CrossRef]
- Kim, D.-H.; Provenzano, P.P.; Smith, C.L.; Levchenko, A. Matrix nanotopography as a regulator of cell function. J. Cell Biol. 2012, 197, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.K.; Darling, E.M.; Kulangara, K.; Guilak, F.; Leong, K.W. Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials 2010, 31, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- McNamara, L.E.; McMurray, R.J.; Biggs, M.J.; Kantawong, F.; Oreffo, R.O.; Dalby, M.J. Nanotopographical control of stem cell differentiation. J. Tissue Eng. Regen. Med. 2010, 1, 120623. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Jiao, A.; Hwang, N.S.; Kim, M.S.; Kim, D.-H.; Suh, K.-Y. Nanotopography-guided tissue engineering and regenerative medicine. Adv. Drug Deliv. Rev. 2013, 65, 536–558. [Google Scholar] [CrossRef] [PubMed]
- Dalby, M.J.; Gadegaard, N.; Tare, R.; Andar, A.; Riehle, M.O.; Herzyk, P.; Wilkinson, C.D.W.; Oreffo, R.O.C. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nat. Mater. 2007, 6, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Shah, S. The nanomaterial tookit for neuroengineering. Nano Converg. 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-C.; Choo, S.-S.; Kim, K.-T.; Hong, K.-S.; Moon, S.-H.; Cha, H.-J.; Kim, T.-H. Conductive hybrid matrigel layer to enhance electrochemical signals of human embryonic stem cells. Sens. Actuator B Chem. 2017, 424, 224–230. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, E.-S.; Kim, D.-S.; Han, Y.; Son, H.; Chung, Y.-H.; Min, J.; Kim, T.-H. Three-Dimensional Graphene–RGD Peptide Nanoisland Composites That Enhance the Osteogenesis of Human Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2018, 19, 669. https://doi.org/10.3390/ijms19030669
Kang E-S, Kim D-S, Han Y, Son H, Chung Y-H, Min J, Kim T-H. Three-Dimensional Graphene–RGD Peptide Nanoisland Composites That Enhance the Osteogenesis of Human Adipose-Derived Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2018; 19(3):669. https://doi.org/10.3390/ijms19030669
Chicago/Turabian StyleKang, Ee-Seul, Da-Seul Kim, Yoojoong Han, Hyungbin Son, Yong-Ho Chung, Junhong Min, and Tae-Hyung Kim. 2018. "Three-Dimensional Graphene–RGD Peptide Nanoisland Composites That Enhance the Osteogenesis of Human Adipose-Derived Mesenchymal Stem Cells" International Journal of Molecular Sciences 19, no. 3: 669. https://doi.org/10.3390/ijms19030669
APA StyleKang, E. -S., Kim, D. -S., Han, Y., Son, H., Chung, Y. -H., Min, J., & Kim, T. -H. (2018). Three-Dimensional Graphene–RGD Peptide Nanoisland Composites That Enhance the Osteogenesis of Human Adipose-Derived Mesenchymal Stem Cells. International Journal of Molecular Sciences, 19(3), 669. https://doi.org/10.3390/ijms19030669